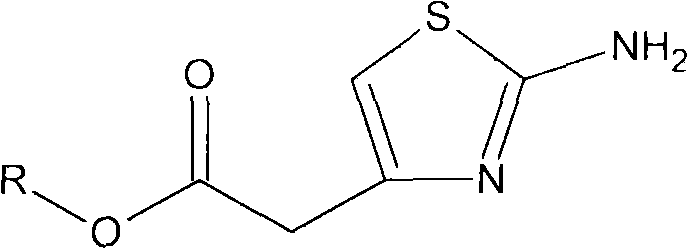
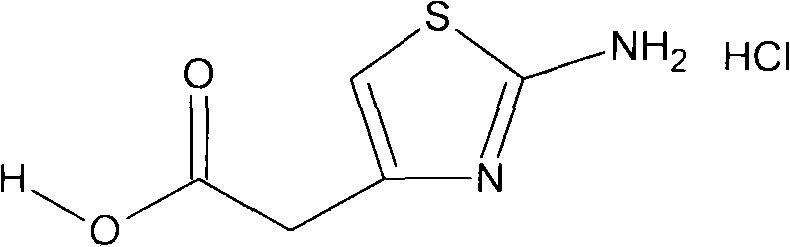
Method for preparing 2-aminothiazol-4-ylacetic acid hydrochloride
A technology of acetic acid hydrochloride and aminothiazole, applied in the field of manufacturing pharmaceutical intermediates, can solve problems such as complicated manufacturing methods, high product cost, unsuitable for industrialization, etc. Handling easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
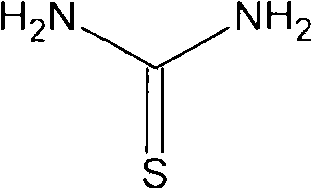
[0032] Suspend 40g of thiourea in 100ml of water, stir for 20 minutes to dissolve and lower the temperature to 0°C, keep the temperature at 0-3°C, drop 68.5ml of ethyl 4-chloroacetoacetate in about 2 hours, after the drop Stir at the same temperature for 3 hours. After the heat preservation is completed, the pH is adjusted to 7 with ammonia water, and white crystals are precipitated, and the intermediate compound of molecular formula 4 is obtained by filtration.
[0033] After cooling 100ml of concentrated hydrochloric acid to 0~3°C, suspend the intermediate compound obtained in the previous step in the prepared cold concentrated hydrochloric acid at this temperature, stir for 60 minutes, then raise the temperature to 60°C, keep it warm for 6 hours, and keep it warm After completion, cool to -5~0°C and filter to obtain 90 g of the target product (chemical formula 1), with a yield of 92% and a purity (HPLC) of 99.5%.
Embodiment 2
[0035] Suspend 60g of thiourea in 160ml of water, stir for 20 minutes to dissolve it and lower the temperature to 0°C, keep the temperature at 1-3°C, drop 132.5ml of ethyl 4-chloroacetoacetate in about 2 hours, after the drop Stir at the same temperature for 3 hours. After the heat preservation is completed, the pH is adjusted to 7 with ammonia water, white crystals are precipitated, and the intermediate compound represented by molecular formula 4 is obtained by filtration.
[0036] After cooling 150ml of concentrated hydrochloric acid to 1-3°C, suspend the intermediate compound obtained in the previous step in the prepared cold concentrated hydrochloric acid at this temperature, stir for 60 minutes, then raise the temperature to 60°C, keep it warm and hydrolyze for 6 hours, After the heat preservation was completed, it was cooled to -3~0°C, and 135.1 g of the target product (chemical formula 1) was obtained by filtration, with a yield of 92% and a purity (HPLC) of 99.6%.
Embodiment 3
[0038] Suspend 100g of thiourea in 250ml of water, stir for 20 minutes to dissolve it and lower the temperature to 0°C, keep the temperature at 0-2°C, drop 171.5ml of ethyl 4-chloroacetoacetate in about 2 hours, after the drop Stir at the same temperature for 3 hours. After the heat preservation is completed, the pH is adjusted to 7 with ammonia water, and white crystals are precipitated, and the intermediate compound of molecular formula 4 is obtained by filtration.
[0039] After cooling 250ml of concentrated hydrochloric acid to 1-2°C, suspend the intermediate compound obtained in the previous step in the prepared cold concentrated hydrochloric acid at this temperature, stir for 60 minutes, then raise the temperature to 60°C, heat-preserve and hydrolyze for 6 hours, and keep warm After completion, cool to -5~-2°C and filter to obtain 228g of the target product (chemical formula 1), with a yield of 92% and a purity (HPLC) of 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com