Improvements in crystallization of an intermediate for synthesizing non-ionic x-ray contrast agents
A crystallization and compound technology, applied in the preparation of organic compounds, semi-permeable membrane separation, separation/purification of carboxylic acid amides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 (comparative example)
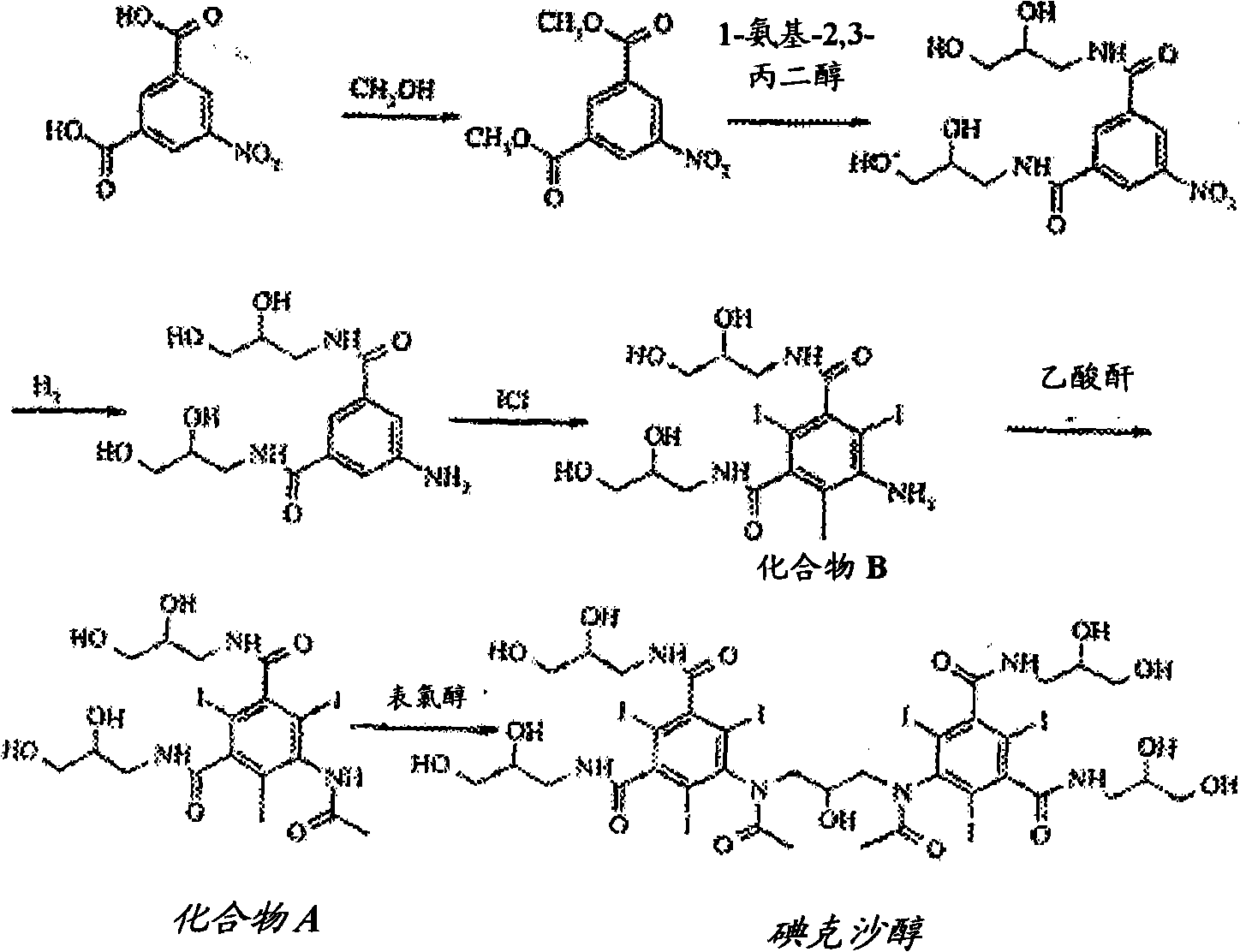
[0023] 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (452 kg) was triiodated with aqueous iodine chloride in an aqueous reaction medium. Excess iodine chloride was quenched by adding sodium bisulfite. Add 5-amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide (compound B) (5.5kg ) seeds. The total amount of water in the mixture is about 3200-3300 liters at this stage. The seeded mixture was cooled slowly to 32°C. Crystal growth was allowed to continue for 12 hours, then filtered and the filter cake washed with water.
Embodiment 2
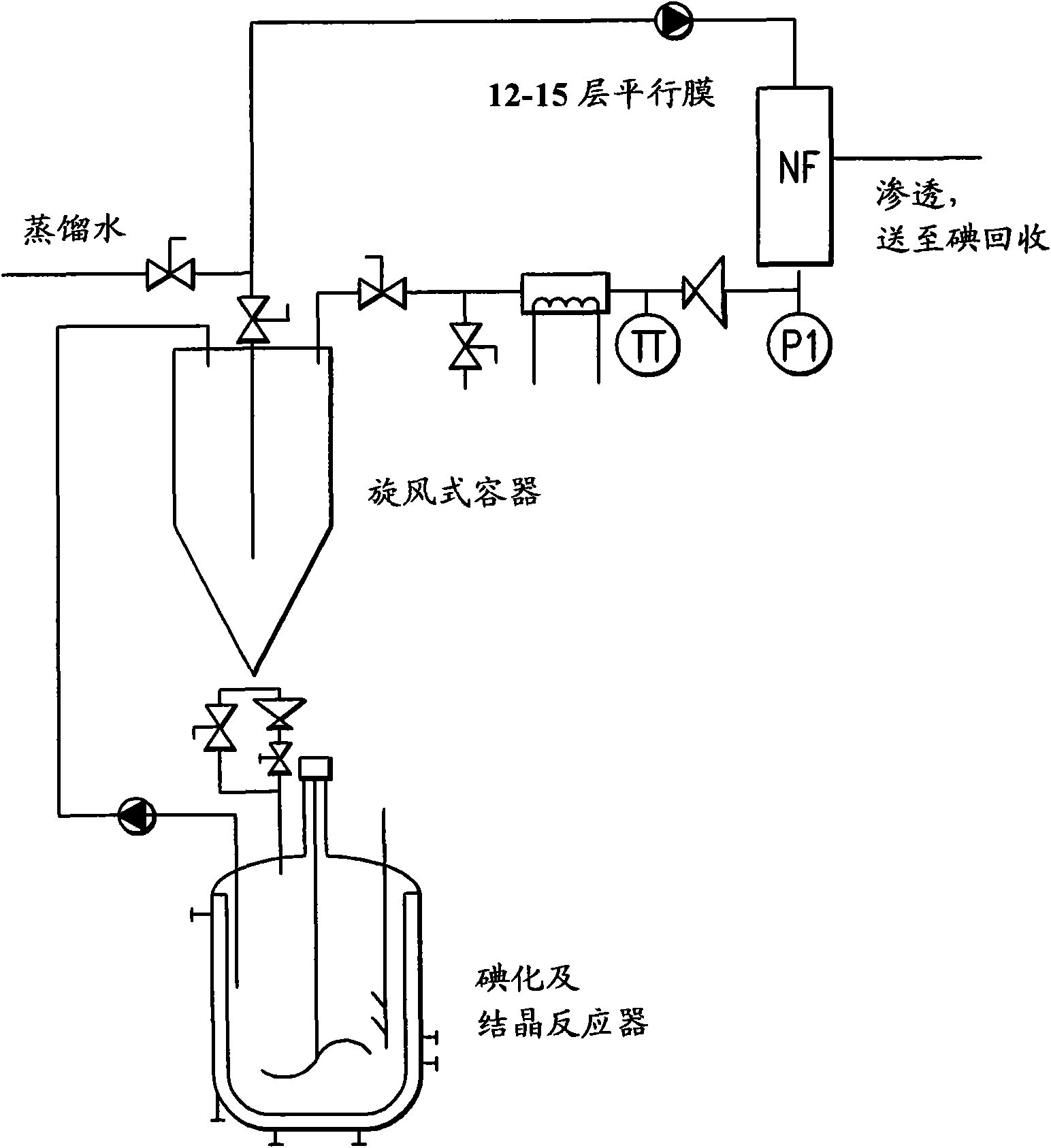
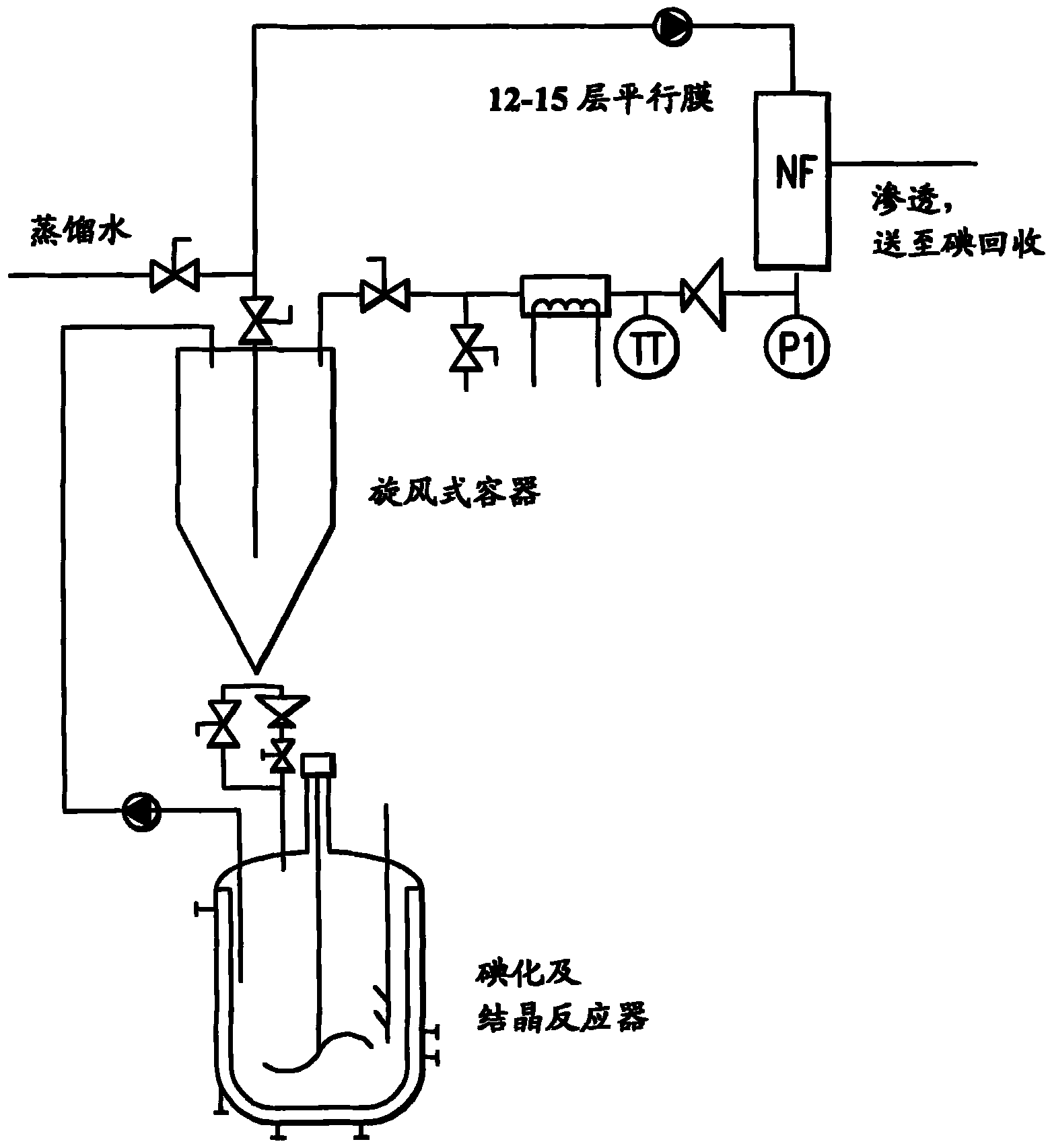
[0025] 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (452 kg) was triiodated with aqueous iodine chloride in an aqueous reaction medium. Excess iodine chloride was quenched by adding sodium bisulfite. Add 5-amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide (compound B) (5.5kg ) seeds. The total amount of water in the mixture is about 3200-3300 liters at this stage. The seeded mixture was cooled slowly to 35°C and subjected to nanofiltration followed by diafiltration with water. The mother liquor was decanted from the slurry and concentrated to about 60% of its original volume in a nanofiltration unit before being returned to the crystallization vessel. Crystal growth was allowed to continue for 12 hours, then filtered and the filter cake washed with water. The isolated yield of Compound B was about 3.5% higher than that in Comparative Example 1.
Embodiment 3
[0027] 5-Amino-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzenedicarboxamide (452 kg) was triiodated with aqueous iodine chloride in an aqueous reaction medium. Excess iodine chloride was quenched by adding sodium bisulfite. Add 5-amino-N, N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide (compound B) (5.5kg ) seeds. The total amount of water in the mixture is about 3200-3300 liters at this stage. The seeded mixture was cooled slowly to 35°C and subjected to nanofiltration followed by diafiltration with water. The mother liquor was decanted from the slurry and concentrated in a nanofiltration unit before it was returned to the crystallization vessel. This step was repeated twice, resulting in an approximately 50% reduction in the total volume of the mother liquor. Crystal growth was allowed to continue for 12 hours, then filtered and the filter cake washed with water. The isolated yield of Compound B was about 4% higher than that in Comparative Example 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap