Malaria vaccine compositions and constituents which elicit cell mediated immunity
A malaria and recombinant antigen technology, applied in the field of malaria vaccines, can solve the problems of unsuccessful malaria vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0043] Example 1, Selection of Variable Epitopes: Analysis of CSP Variable Regions
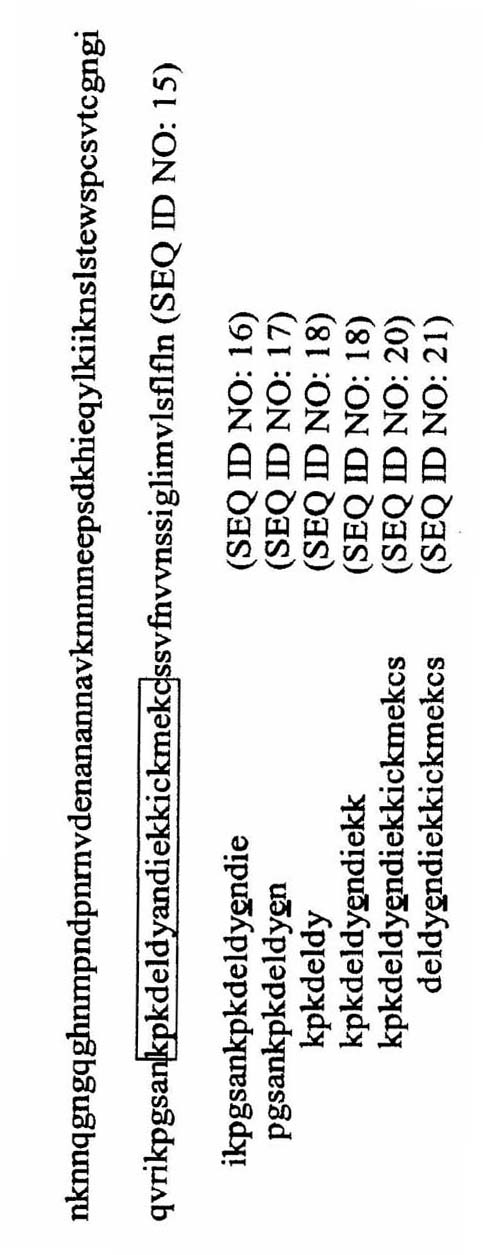
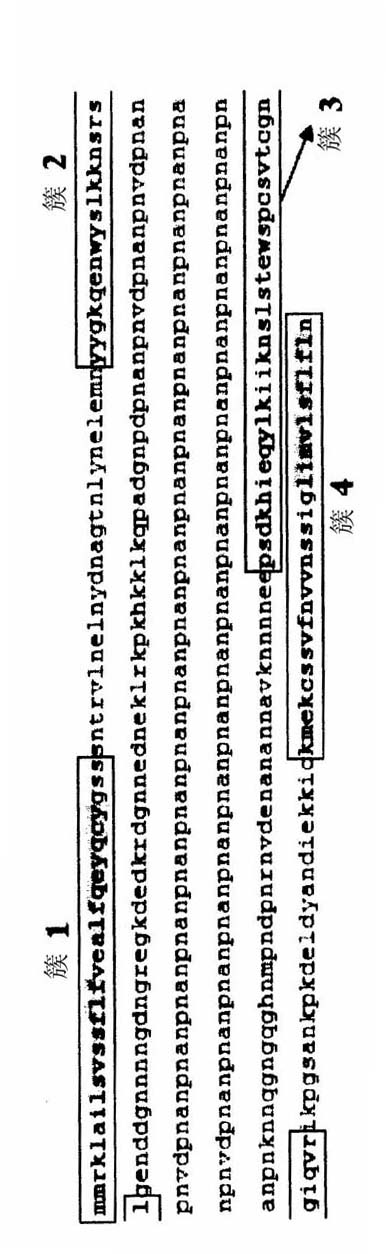
[0044] Variable regions 288 to 412 of the CSP protein from Plasmodium falciparum 3D7 ( image 3 The analysis of SEQ ID NO:15) was performed according to the IEDB database to determine whether the similarities and differences between the sequences were T cell antigenic determinants. Recognized T cell epitopes such as image 3 Shown in SEQ ID NO:16-21. The analysis results showed that the 22 amino acid sequences recognized in this variable region (residues 368-389 of SEQ ID NO: 15, in image 3 The CSP sequence in the box, numbered as SEQ ID NO: 38) contains an important part of the T cell epitope, which can be used to query the NCBI database to identify the peptide containing the 22 amino acids shown (KPKDELDYANDIEKKICKMEKC, SEQ ID NO: 38) Proteins with highly related amino acid sequences. These sequences with high identity were further analyzed by IEDB. The results are listed in Table 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com