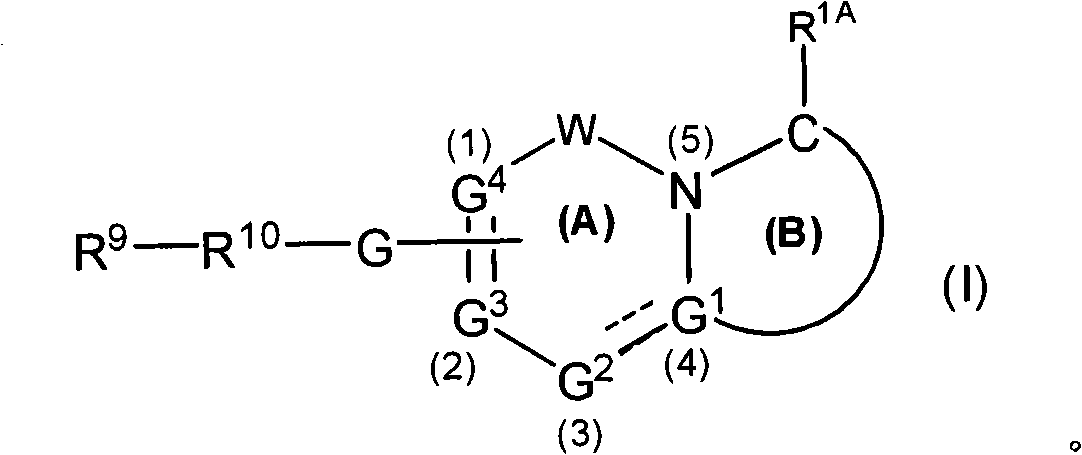
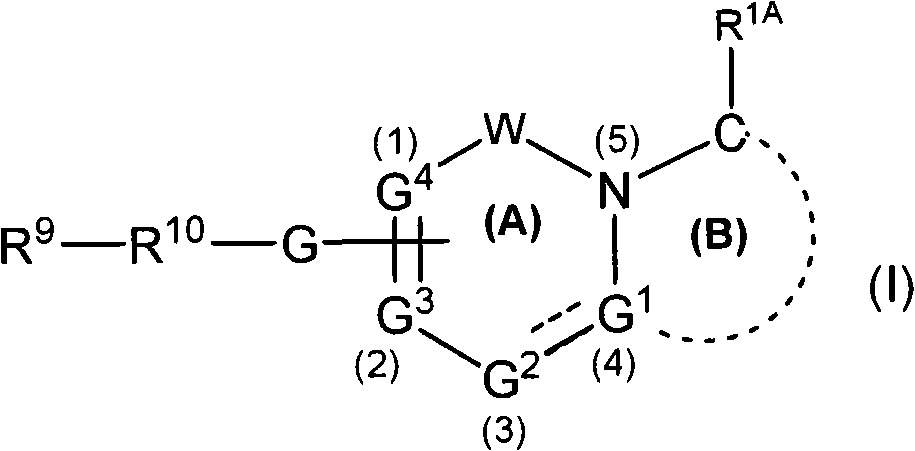
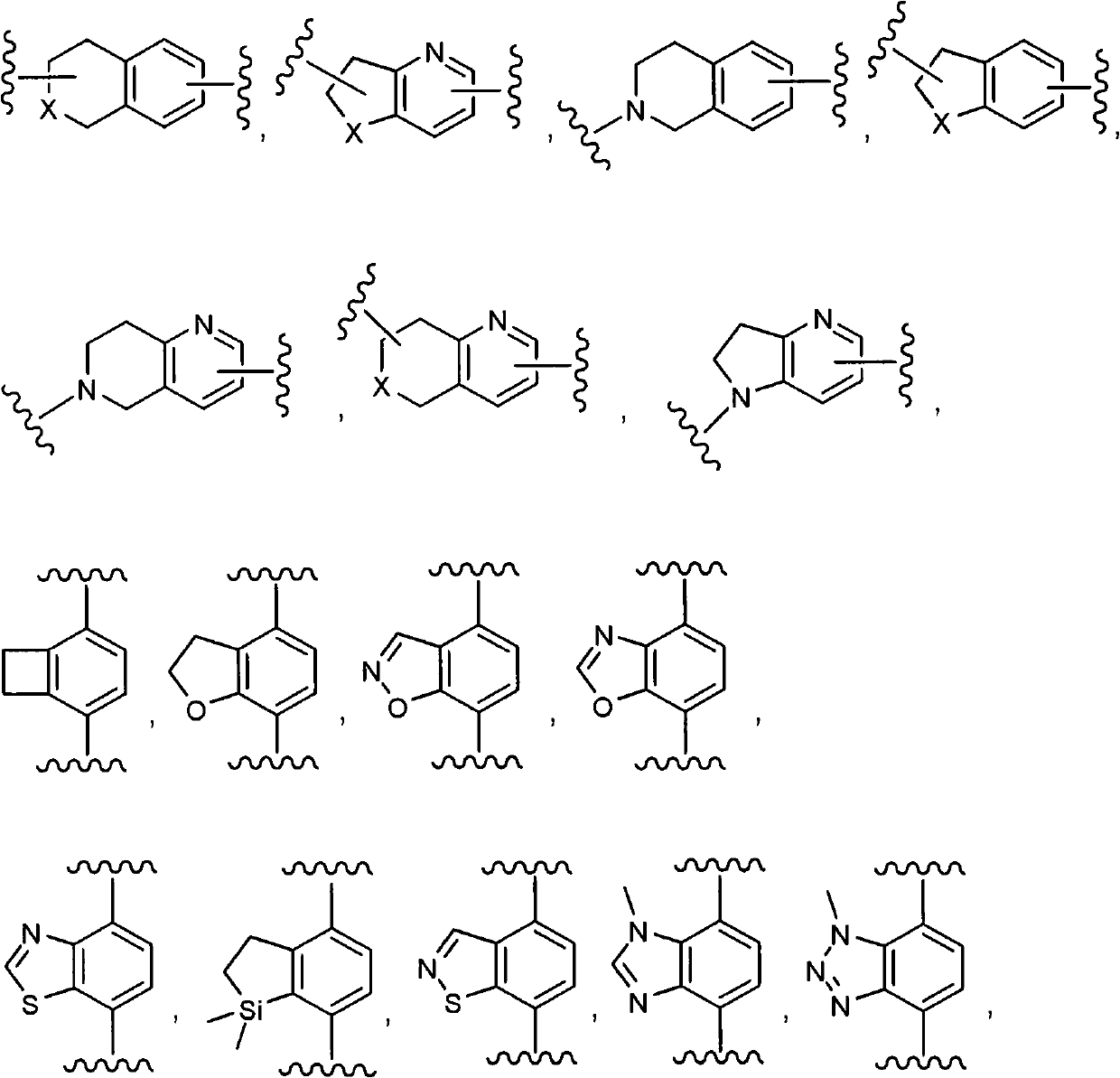
Gamma secretase modulators
A solvate, pharmaceutical technology, applied in the field of gamma secretase regulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[1993]
[1994] Step A:
[1995]
[1996] Compound E2a (2.03 g, 10 mmol), Cu 2 O (0.288 g, 2 mmol), PEG (4.0), Cs 2 CO 3 (9.77 g, 30 mmol), a mixture of 4-methylimidazole (0.98 g, 12 mmol) and E2b (0.72 g, 3 mmol) in NMP (15 mL) was vacuumed at 120 °C in a sealed tube - Nitrogen exchange degassing and stirring for 48 hours. The mixture was cooled to room temperature and washed with CH 2 Cl 2 Dilute, then add silica gel. The mixture was stirred for 20 minutes and filtered. The organic layer was washed with water (3x), brine, washed over MgSO 4 Drying, and concentration gave crude product. The crude residue was purified by column chromatography with CH 2 Cl 2 Elution with / MeOH afforded compound E2c (0.2 g).
[1997] Step B:
[1998]
[1999] Compound E1h (0.141 mmol), E2c (28.8 mg, 0.141 mmol), potassium carbonate (0.117 g, 0.846 mmol) and CuBr.Me 2 A mixture of S (58 mg, 0.282 mmol) in pyridine (1.0 mL) was heated at 140°C overnight. The mixture was wash...
Embodiment 3
[2001]
[2002] Step A:
[2003]
[2004] To a solution of compound E1h (1 equiv) in THF was added 1 PrMgCl.LiCl (1M in THF, 1.3 equiv) and stir for 20 minutes. This was followed by the addition of methyl cyanoformate (1.0 equiv). The resulting mixture was stirred at room temperature for 2 h, after which it was washed with saturated aqueous NH 4 Cl diluted, will be extracted with EtOAc, will be dried (Na 2 SO 4 ), will be concentrated and will be purified by silica gel flash chromatography (Hex / EtOAc) to give compound E3a.
[2005] Step B:
[2006]
[2007] E3a will be hydrolyzed with LiOH in water / MeOH / THF to give E3b.
[2008] Step C:
[2009]
[2010] Compounds E1j (0.596mmol) and E3b (0.596mmol) will be mixed into DCM (4ml) at room temperature, which is then added with HOBT (96mg, 0.715mmol), EDC (136mg, 0.715mmol) and DIEA (300μl, 1.2mmol). The resulting mixture was continuously stirred at room temperature for 16 hours. The mixture will use CH 2 Cl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com