Method for recycling vanadium pentoxide and sodium dichromate
A technology of vanadium pentoxide and sodium dichromate, applied in the field of chromium salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
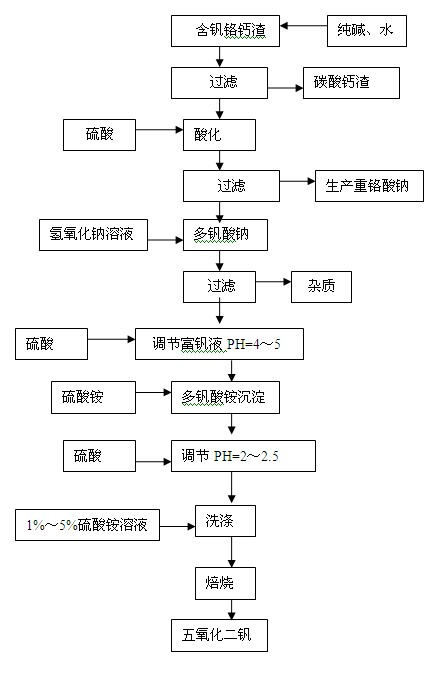
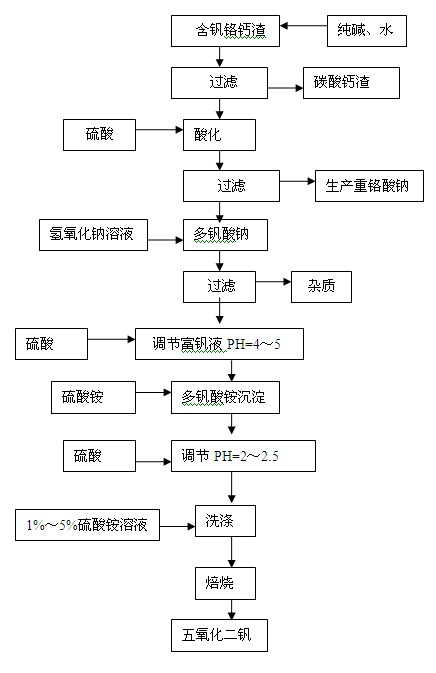
[0033] Embodiment 1: reclaim sodium dichromate,
[0034] Take 1.8 kg of vanadium-containing, chromium-calcium slag (Na 2 Cr 2 o 7 .2H 2 O: 13%, V 2 o 5 : 1.63% CaO: 28%), add 1.8 kg of water, beating and stirring evenly, then add 0.36 kg of soda ash, stir and leach at 80°C for 2 hours. Filter calcium carbonate slag, in alkaline solution (Na 2 Cr 2 o 7 .2H 2 O: 148g / L, V 2 o 5 : 15g / L) Slowly add sulfuric acid to adjust PH = 1.2, heat and mature at 90°C for 1 hour, filter and wash the sodium polyvanadate precipitate (V 2 o 5 : 53%, Na 2 Cr 2 o 7 .2H 2 O: 2.8%); acidic sodium dichromate solution (PH=1.2, V 2 o 5 : 0.45g / L, Na 2 Cr 2 o 7 .2H 2 O: 124g / L) into the sodium dichromate production line to produce sodium dichromate.
Embodiment 2
[0035] Embodiment 2: reclaim vanadium pentoxide,
[0036] Get the sodium polyvanadate precipitation of embodiment 1 filtration, wash and be 4% industrial sodium hydroxide heating and dissolving with 600ml concentration and adjust PH=8, filter to remove impurity, add sulfuric acid 1ml in the vanadium-rich liquid, adjust PH to be 2, Add 36 grams of industrial ammonium sulfate, stir at 50°C for 0.8 hours, filter and precipitate ammonium polyvanadate, wash with 600ml of ammonium sulfate with a concentration of 3% for 4 times on average, and the hexavalent chromium in the last washing solution is 0.6g / L. The ammonium polyvanadate precipitate is dried at 105-110°C for 2 hours, put into a resistance furnace and roasted at 550°C for 2 hours to produce vanadium pentoxide with a content of 99.68%.
Embodiment 3
[0038] Take 1.5 kg of vanadium-containing calcium slag (Na 2 Cr 2 o 7 .2H 2 O: 12%, V 2 o 5 : 1.5% CaO: 30%), add 1.5 kg of water, beating and stirring evenly, then add 0.15 kg of soda ash, stir and leach at 85°C for 3 hours, and filter the calcium carbonate residue. Its alkaline solution (Na 2 Cr 2 o 7 .2H 2 O: 136.8g / L, V 2 o 5 : 16.8g / L) with sulfuric acid to adjust PH=2.5, 90 ℃ heat preservation and aging for 3 hours, filter and wash the sodium polyvanadate precipitate (V 2 o 5 : 59.28%, Na 2 Cr 2 o 7 .2H 2 O: 2.5%); acidic sodium dichromate solution (PH=2.5, V 2 o 5 : 0.80g / L, Na 2 Cr 2 o 7 .2H 2O: 130g / L) to produce sodium dichromate. Heat and dissolve sodium polyvanadate with 250ml of 7% sodium hydroxide, adjust the pH to 9, and filter to remove impurities. Add 0.8ml of sulfuric acid to the vanadium-enriched liquid, adjust PH=2.5, add 39.2 grams of industrial ammonium sulfate, stir at 60°C for 0.5 hours, filter, and wash the resulting ammonium po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com