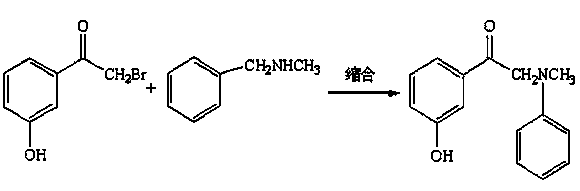
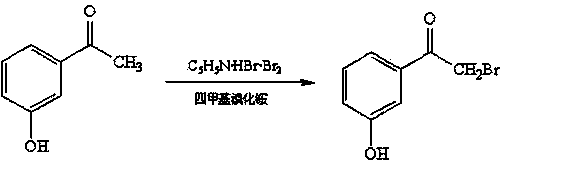
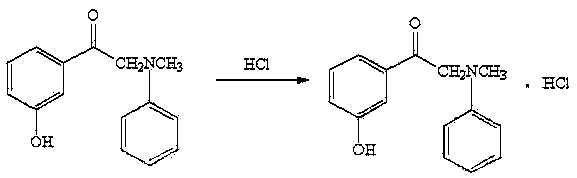
Preparation and refining method of alpha-(N-benzyl-N-methyl amino)-m-hydroxyacetophenone hydrochloride
The technology of hydroxyacetophenone hydrochloride and m-hydroxyacetophenone is applied in the field of preparation and refining of α--m-hydroxyacetophenone hydrochloride, and can solve the problem of difficult post-processing, long reaction steps and environmental pollution Small problems, etc., to achieve the effect of being conducive to solvent recovery, low cost, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis and purification of embodiment 1α-(N-benzyl-N-methylamino)-m-hydroxyacetophenone hydrochloride
[0026] Petroleum ether: 400kg
[0027] m-Hydroxyacetophenone: 68kg
[0028] Tetramethylammonium bromide: 0.7kg
[0029] C 5 h 5 N·HBr·Br 2 : 80kg
[0030] Sodium carbonate: 30kg
[0031] N-methylbenzylamine: 121kg
[0032] Hydrochloric acid: 100kg
[0033] Ethanol: 300kg
[0034] (1) Catalyzed bromination reaction:
[0035] In a 1500L reactor, put 400kg of petroleum ether and 68kg of m-hydroxyacetophenone, add 0.7kg of catalyst tetramethylammonium bromide, stir for 0.5h after warming up to room temperature, add brominating agent C 5 h 5 N·HBr·Br 2 80kg, about 1h, the generated hydrogen bromide waste gas is absorbed with lye, after the dropwise addition, keep warm for 2h, and the bromide reaction liquid is obtained.
[0036] (2) Amination condensation reaction:
[0037] Transfer the above bromination reaction solution to the amination kettle, add 50kg...
Embodiment 2
[0042] Synthesis and purification of embodiment 2α-(N-benzyl-N-methylamino)-m-hydroxyacetophenone hydrochloride
[0043] Petroleum ether: 400kg
[0044] m-Hydroxyacetophenone: 70kg
[0045] Tetramethylammonium bromide: 1.1kg
[0046] C 5 h 5 N·HBr·Br 2 : 90kg
[0047] Sodium carbonate: 30kg
[0048] N-methylbenzylamine: 130kg
[0049] Hydrochloric acid: 120kg
[0050] Ethanol: 310kg
[0051] (1) Catalyzed bromination reaction:
[0052] In a 1500L reactor, put 400kg of petroleum ether and 70kg of m-hydroxyacetophenone, add 1.1kg of catalyst tetramethylammonium bromide, stir for 0.5h after warming up to room temperature, add brominating agent C 5 h 5 N·HBr·Br 2 90kg, about 1h, the generated hydrogen bromide waste gas is absorbed with lye, and after the dropwise addition, keep warm for 3h to obtain the bromide reaction liquid.
[0053] (2) Amination condensation reaction:
[0054] Transfer the above bromination reaction solution to the amination kettle, add 50kg of w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com