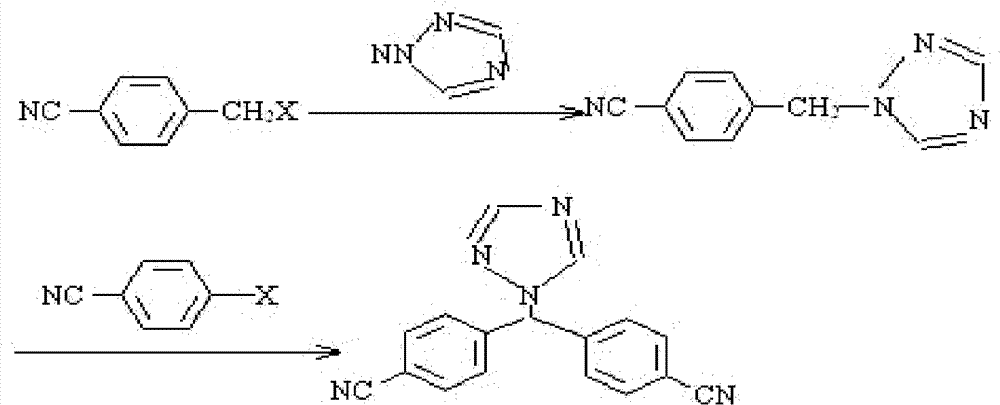
Method for synthesizing trazodone
A synthetic method, letrozole technology, applied in the field of drug synthesis, can solve the problems of difficult removal of impurities, low purity, low yield of letrozole, etc., and achieve the effect of being suitable for large-scale production and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
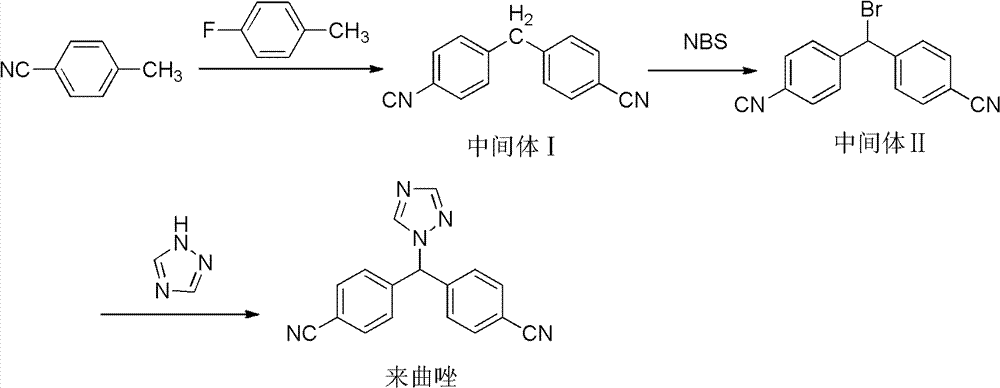
[0025] Add 500mL tetrahydrofuran dried over anhydrous magnesium sulfate into a 2000mL reaction bottle, add 36g sodium methoxide under nitrogen protection under stirring, cool down to below 0°C in an ice-salt bath, slowly add 117g p-toluonitrile and 125g p-fluorobenzonitrile tetrahydrofuran dropwise Mix the solution and keep it below 0°C for 2 hours after dropping. Wash 3 times with 300 mL of saturated brine, dry over anhydrous sodium sulfate, distill THF off, and crystallize the residue with methyl tert-butyl ether to obtain 113 g of Intermediate I, melting point: 168.5°C, HPLC purity: 99.7%
[0026] 113g of intermediate I obtained above was dissolved in 600mL of chloroform, 92g of N-bromosuccinimide and 4g of azobisisobutyronitrile were added in sequence, the mixture was heated to reflux temperature and kept at this temperature for 9-10h. The reaction solution was cooled to room temperature, and the chloroform layer was washed three times with 300 mL of saturated saline, drie...
Embodiment 2
[0029] Synthesis of 4,4'-cyanodiphenylmethane
[0030] Add 500mL of tetrahydrofuran dried over anhydrous magnesium sulfate into a 2000mL reaction bottle, add 32.5g of sodium methoxide under stirring and nitrogen protection, cool down to below 5°C in an ice-salt bath, slowly add 117g of p-toluonitrile and 133g of p-fluorobenzonitrile dropwise The mixed solution of tetrahydrofuran was kept below 0°C for 2 hours after dropping. Wash 3 times with 300 mL of saturated brine, dry over anhydrous sodium sulfate, evaporate THF, and crystallize the residue with methyl tert-butyl ether to obtain 109 g of intermediate I, melting point: 168.9 °C, HPLC purity: 98.2%
[0031] Synthesis of 4-(α-bromo-4-cyano)benzonitrile
[0032] 113g of intermediate I was dissolved in 600mL of chloroform, 100g of N-bromosuccinimide and 2g of azobisisobutyronitrile were added in sequence, the mixture was heated to reflux temperature and kept at this temperature for 9-10h. The reaction solution was cooled to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com