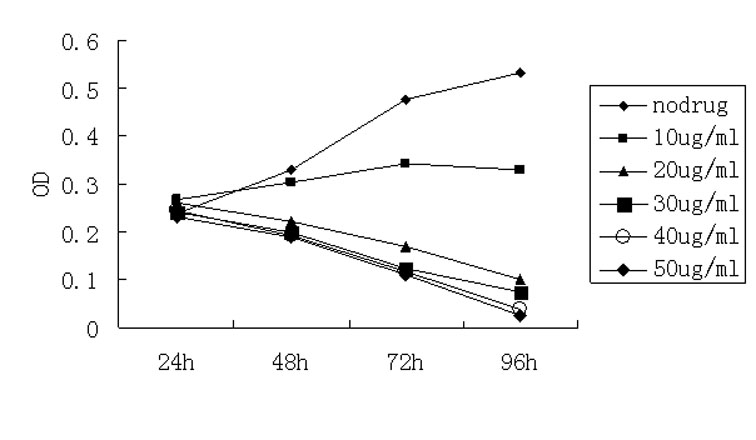
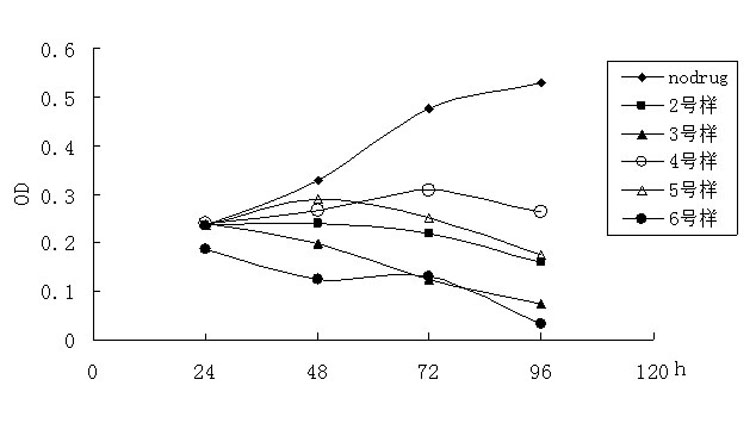
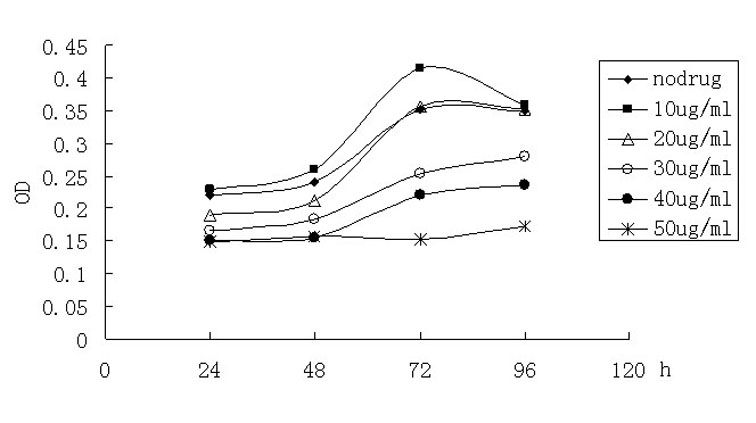
Nitroxyl radical anti-tumor medicaments
A drug and selected technology, applied in the field of medicine, can solve problems such as large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the synthetic method of compound 1
[0020]
[0021] Add 3.0 g (30 mmol) prolinol, CH 2 Cl 2 100 mL, 4.4 mL of triethylamine were magnetically stirred in an ice bath, and then added dropwise to 50 mL of CH containing 5.57 g (30 mmol) p-nitrobenzoyl chloride 2 Cl 2 solution. After the dropwise addition, the ice bath was removed, stirred at room temperature for 24 h, the organic phase was separated, washed twice with water, dried, and purified by column chromatography to obtain 6.4 g of the product.
[0022] The above product 1.0 g (4.0 mmol), 0.93 g (4.0 mmol) TCCA and 20 mL CH 2 Cl 2 , stirred at 0 °C for 5 min, then added 0.006 g (0.04 mmol) TEMPO. Stirring was continued at room temperature for 20 min. Filter, combine the organic phases, and wash with saturated Na 2 CO 3 Wash with 0.1 mol·L -1 The organic phase was washed with HCl and saturated brine. The organic phase was dried and purified by column chromatography to obtain 0.69 g of t...
Embodiment 2
[0024] Embodiment 2: the synthetic method of compound 2
[0025]
[0026] Add 3.0 g (30 mmol) prolinol, CH 2 Cl 2 100 mL, 4.4 mL of triethylamine were magnetically stirred in an ice bath, and then added dropwise to 50 mL of CH containing 4.74 g (30 mmol) p-fluorobenzoyl chloride 2 Cl 2 solution. After the dropwise addition, the ice bath was removed, stirred at room temperature for 24 h, the organic phase was separated, washed twice with water, dried, and purified by column chromatography to obtain 5.5 g of the product.
[0027] 0.89 g (4.0 mmol) of the above product, 0.93 g (4.0 mmol) TCCA and 20 mL CH 2 Cl 2 , stirred at 0 °C for 5 min, then added 0.006 g (0.04 mmol) TEMPO. Stirring was continued at room temperature for 20 min. Filter, combine the organic phases, and wash with saturated Na 2 CO 3 Wash with 0.1 mol·L -1 The organic phase was washed with HCl and saturated brine. The organic phase was dried and purified by column chromatography to obtain 0.69 g...
Embodiment 3
[0029] Embodiment 3: the synthetic method of compound 3
[0030]
[0031] Add 3.0 g (30 mmol) prolinol, CH 2 Cl 2 100 mL, 4.4 mL of triethylamine were magnetically stirred in an ice bath, and then added dropwise to 50 mL of CH containing 4.68 g (30 mmol) p-chlorobenzoyl chloride 2 Cl 2 solution. After the dropwise addition, the ice bath was removed, stirred at room temperature for 24 h, the organic phase was separated, washed twice with water, dried, and purified by column chromatography to obtain 5.8 g of the product.
[0032] 0.88 g (4.0 mmol) of the above product, 0.93 g (4.0 mmol) TCCA and 20 mL CH 2 Cl 2 , stirred at 0 °C for 5 min, then added 0.006 g (0.04 mmol) TEMPO. Stirring was continued at room temperature for 20 min. Filter, combine the organic phases, and wash with saturated Na 2 CO 3 Wash with 0.1 mol·L -1 The organic phase was washed with HCl and saturated brine. The organic phase was dried and purified by column chromatography to obtain 0.78 g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com