O/w type matrine percutaneous absorption microemulsion
A matrine and microemulsion technology, applied in the field of medicine, can solve the problems of large fluctuation of blood drug concentration, short drug half-life, inconvenience, etc., and achieve the effects of improving bioavailability, stable blood drug concentration, and overcoming short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0010] [Preparation of Microemulsion]
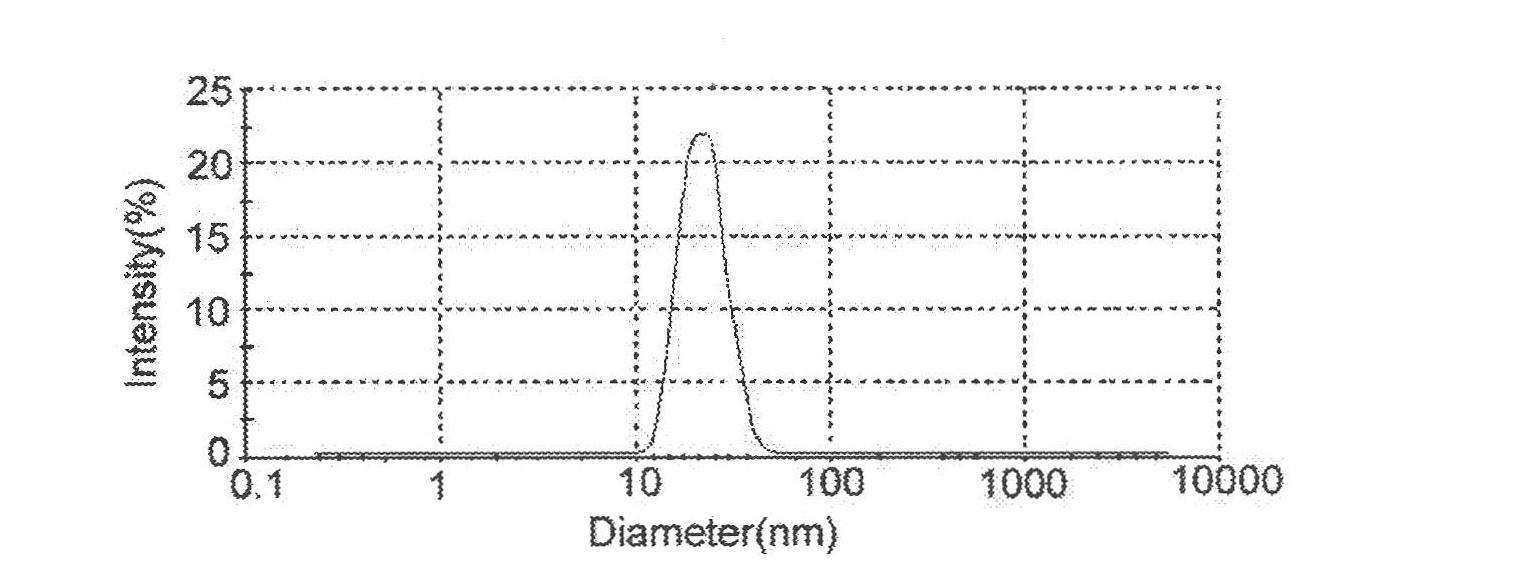
[0011] The O / W type matrine transdermal absorption microemulsion is composed of matrine and matrix. The matrix is composed of four components: surfactant, co-surfactant, oil phase and water. Wherein the oil phase is selected from: oleic acid, ethyl oleate, ethyl butyrate; surfactant is selected from: Tween 80, EL 35 (EL), OP (10); co-surfactant selected from: PEG-400, ethanol; water is deionized water. According to the influence of various substrates on the solubility and skin penetration rate of matrine, the oil phase is selected as: ethyl oleate-ethyl butyrate (4:0.5); the surfactant is: EL 35-OP10 (1:1 ); Co-surfactant is: PEG-400. Draw the pseudo ternary phase diagram, use the simple grid method to optimize the microemulsion formulation, and use the microemulsion particle size, uniformity, high skin penetration rate, and less surfactant and co-surfactant dosage as the indicators. The optimized prescription is: matrine 7% (g / g)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap