Method for preparing dairy cow sex control sperm
A technology for sperm and dairy cows, which is applied to the field of preparation of sperm for sex control of dairy cows, can solve the problems of lipid peroxidation, sperm acrosome membrane damage, sperm damage, etc., and achieves the effects of improving fertilization ability, improving sperm quality and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
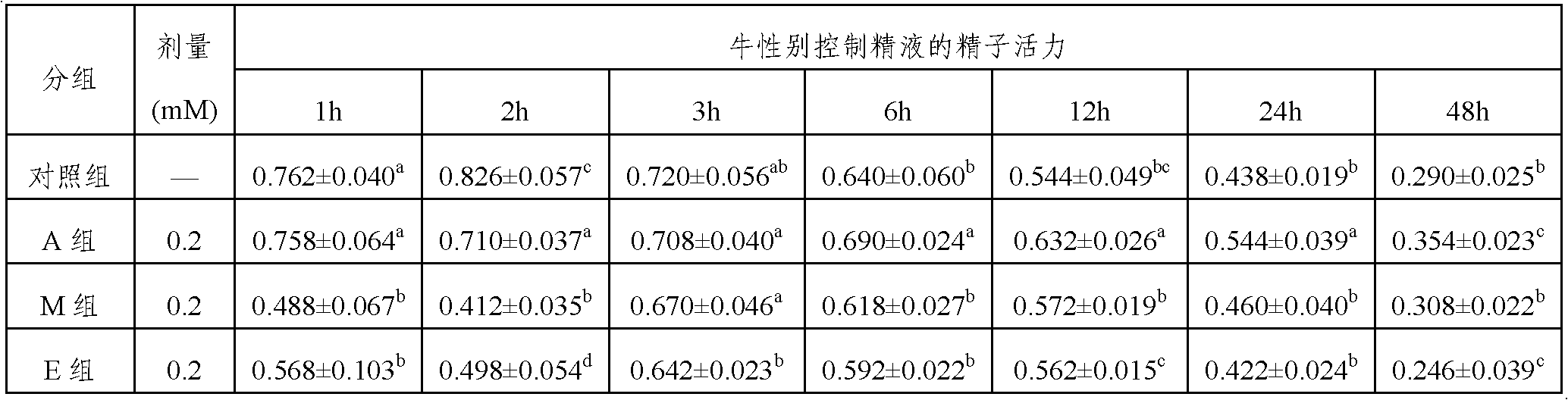
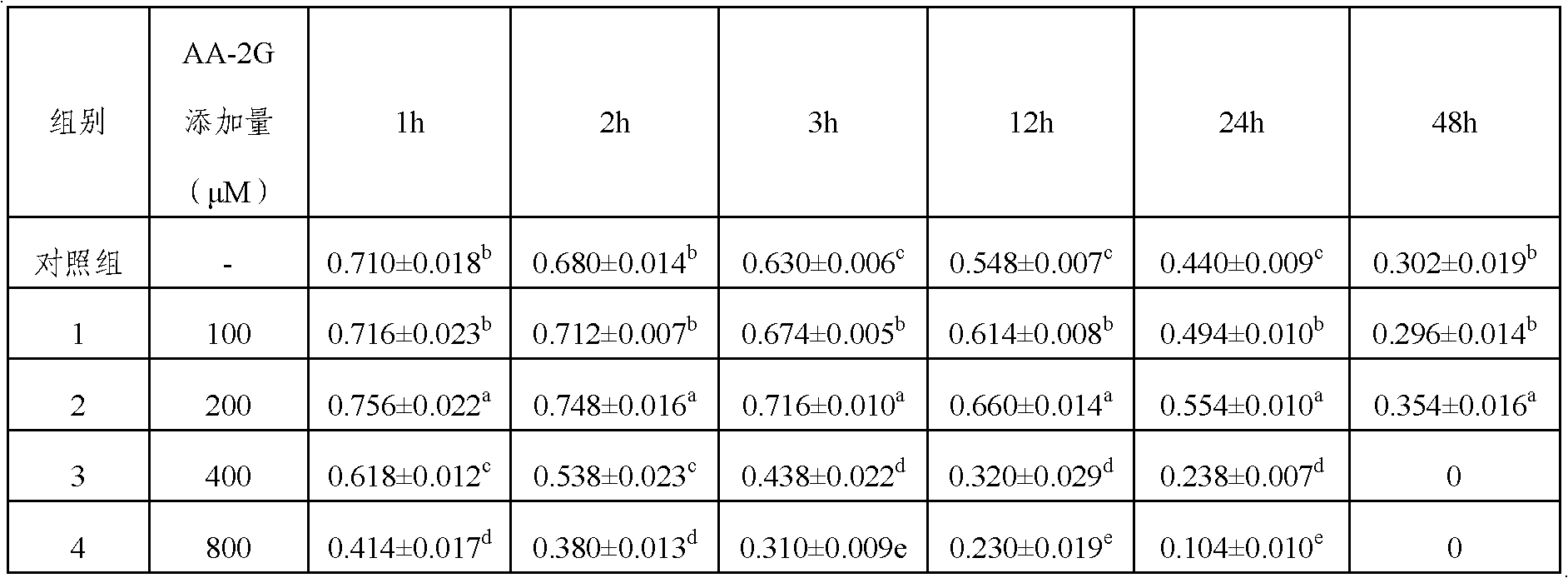
[0024] 1. Take a certain amount of raw cow semen and dilute it with Hepes buffer to 200×10 6 Sperm / mL, then add H33342 to a final concentration of 0.11mM and AA-2G to a final concentration of 150μM, stain in a 34°C water bath for 45min;
[0025] 2. Further dilute to 100×10 with the Hepes damping solution containing 4% (weight to volume ratio) of purified egg yolk and 5% (weight to volume ratio) food red 6 Sperm / mL;
[0026] 3. Then use the SX-MoFlo flow cytometer to separate and recover the sperm, and the separated semen is collected in the frozen I solution containing 250 μM AA-2G; when the sperm is collected to 8×10 6 For sperm, immediately centrifuge at 840g for 20min and discard the supernatant;
[0027] 4. Re-suspend the pellet with frozen I solution containing AA-2G, so that the sperm density per tube is 3×10 6 Sperm were packed in 0.25ml thin tubes, sealed and stored at 15°C.
Embodiment 2
[0029] 1. Take a certain amount of raw cow semen and dilute it with Hepes buffer to 200×10 6 Sperm / mL, then add H33342 to a final concentration of 0.11mM and AA-2G to a final concentration of 250μM, stain in a 34°C water bath for 45min;
[0030] 2. Further dilute to 100×10 with the Hepes damping solution containing 4% (weight to volume ratio) of purified egg yolk and 5% (weight to volume ratio) food red 6 Sperm / mL;
[0031] 3. Then use the SX-MoFlo flow cytometer to separate and recover the sperm, and collect the separated semen in the frozen I solution containing 150 μM AA-2G; when the sperm is collected to 8×10 6 For sperm, immediately centrifuge at 840g for 20min and discard the supernatant;
[0032] 4. Re-suspend the pellet with frozen I solution containing AA-2G, so that the sperm density per tube is 3×10 6 Sperm were packed in 0.25ml thin tubes, sealed and stored at 15°C.
Embodiment 3
[0034] 1. Take a certain amount of raw cow semen and dilute it with Hepes buffer to 200×10 6 Sperm / mL, then add H33342 to a final concentration of 0.11mM and AA-2G to a final concentration of 200μM, stain in a 34°C water bath for 45min;
[0035] 2. Further dilute to 100×10 with the Hepes damping solution containing 4% (weight to volume ratio) of purified egg yolk and 5% (weight to volume ratio) food red 6 Sperm / mL;
[0036]3. Then use the SX-MoFlo flow cytometer to separate and recover the sperm, and the separated semen is collected in the frozen I solution containing 200 μM AA-2G; when the sperm is collected to 8×10 6 For sperm, immediately centrifuge at 840g for 20min and discard the supernatant;
[0037] 4. Re-suspend the pellet with frozen I solution containing AA-2G, so that the sperm density per tube is 3×10 6 Sperm were packed in 0.25ml thin tubes, sealed and stored at 15°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com