Medicine controlled timing-released calcium phosphate cement powder containing various medicines
A technology of calcium phosphate bone cement and medicine, applied in the field of biomedical materials and medicine, can solve the problem that calcium phosphate bone cement is difficult to meet the clinical application, and achieve the effect of promoting bone healing, promoting bone tissue growth and preventing osteolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The calcium phosphate bone cement powder containing multiple drugs in the drug-controlled time-sequential release of this example is prepared by the following method:
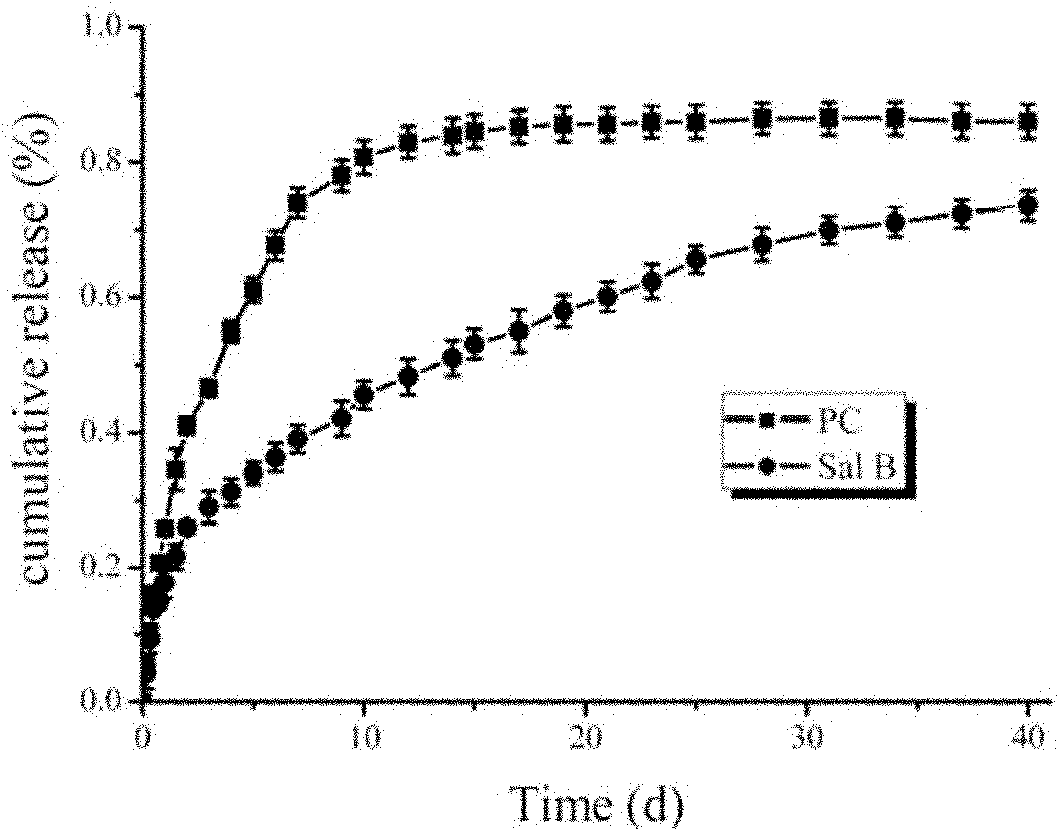
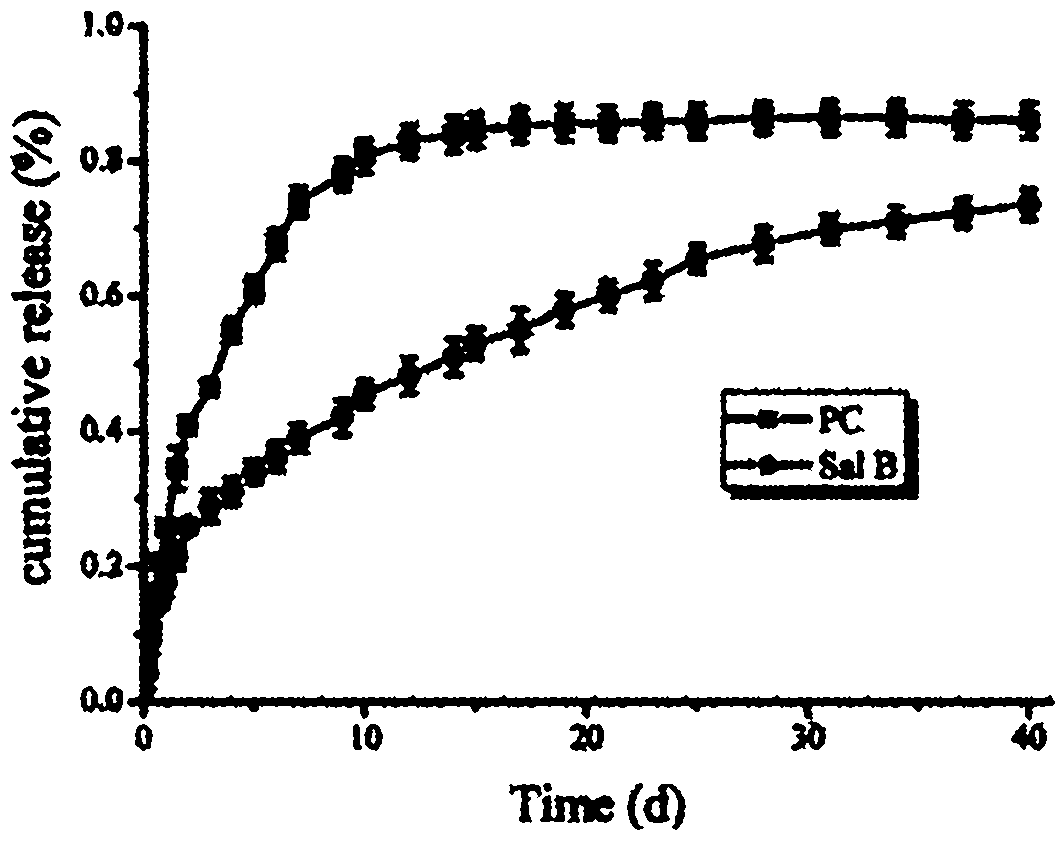
[0031] In the process of wet synthesis of hydroxyapatite (HA) containing 7 grams of dry matter, a long-term release drug containing 1 gram of dry matter was added to the uniformly dispersed suspension—a traditional Chinese medicine that can promote blood circulation and promote bone tissue growth Salvianolic acid B from Salvia miltiorrhiza extract, mix evenly; then add gelatin containing 0.5 g of dry matter, after mixing evenly, add an excessive amount of cross-linking agent - glutaraldehyde to fully cross-link the gelatin, thereby utilizing the macromolecule - gelatin to The drug-loaded hydroxyapatite is precipitated for coating, then dried and ground to obtain the coating powder.
[0032] Take 8.5 grams of wrapping powder containing salvianolic acid B prepared in the previous step, combine with 24 gram...
Embodiment 2
[0036] The calcium phosphate bone cement powder containing multiple drugs in the drug-controlled time-sequential release of this example is prepared by the following method:
[0037] In the process of wet synthesis of calcium dihydrogen phosphate powder with a dry matter amount of 27 grams, 12 grams of long-term release drug-adriamycin and 13 grams of long-term release drug-drynaria fortunei were added to the suspension, and mixed evenly. Then dry the suspension blended by precipitation and grind the powder, add it to the solvent containing 10 grams of polyglycolide, which is a macromolecule for wrapping, and mix evenly, and use polyglycolide to treat the drug-loaded calcium dihydrogen phosphate wrapping, drying and grinding to obtain the medicine-containing wrapping powder.
[0038] Get a total of 62 grams of the wrapping powder prepared in the previous step, and mix them evenly with 9 grams of hydroxyapatite, 64 grams of β-type tricalcium phosphate, 7 grams of sufentanil and...
Embodiment 3
[0041] The calcium phosphate bone cement powder containing multiple drugs in the drug-controlled time-sequential release of this example is prepared by the following method:
[0042] In the process of wet synthesis of calcium carbonate containing 40 grams of dry matter, add long-term release medicine to the suspension-the Chinese medicine ligustrazine extract of 20 grams of dry matter, after fully mixing, add 10 grams of glucose, and add excess Glucose cross-linking agent—formaldehyde fully cross-links the glucose, then dries and grinds the wrapping powder, then adds the wrapping powder to a solvent of 10 grams of degradable polymer polyhydroxybutyrate for secondary wrapping, and finally dries, Grind it into powder to get the wrapping powder for secondary wrapping.
[0043] Take a total of 80 grams of the wrapping powder prepared in the previous step, add 10 grams of hydroxyapatite powder, 50 grams of β-type tricalcium phosphate powder, and release drugs in the early stage—5 g...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap