Antithrombotic medicament for intravenous injection and preparation method and application thereof
A technology of antithrombotic drug and general formula, which is applied in the field of antithrombotic drug for intravenous injection and its preparation, can solve the problems of low purity and poor drug activity, achieve high purity, low degree of polymerization, and inhibit platelet aggregation and adhesion The effect of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Preparation of oligomannuronic acid methyl sulfate sodium salt
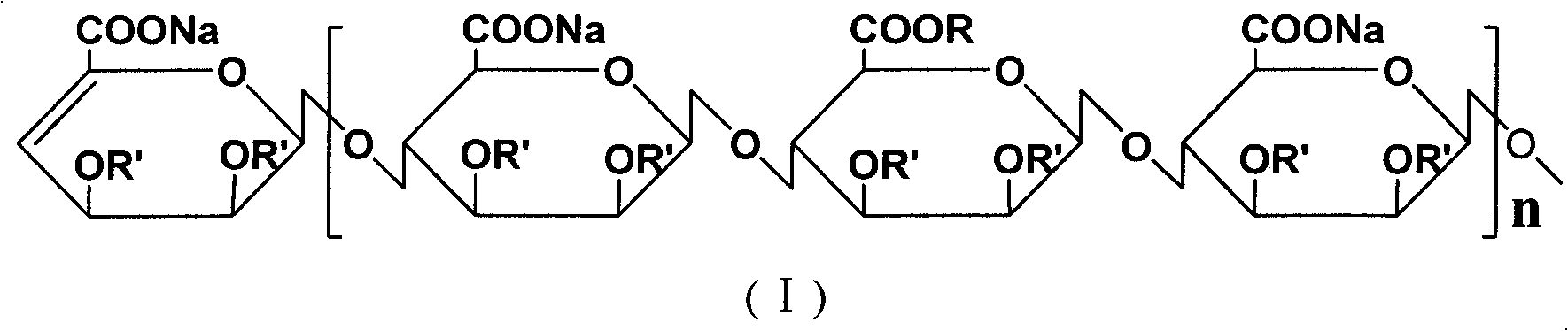
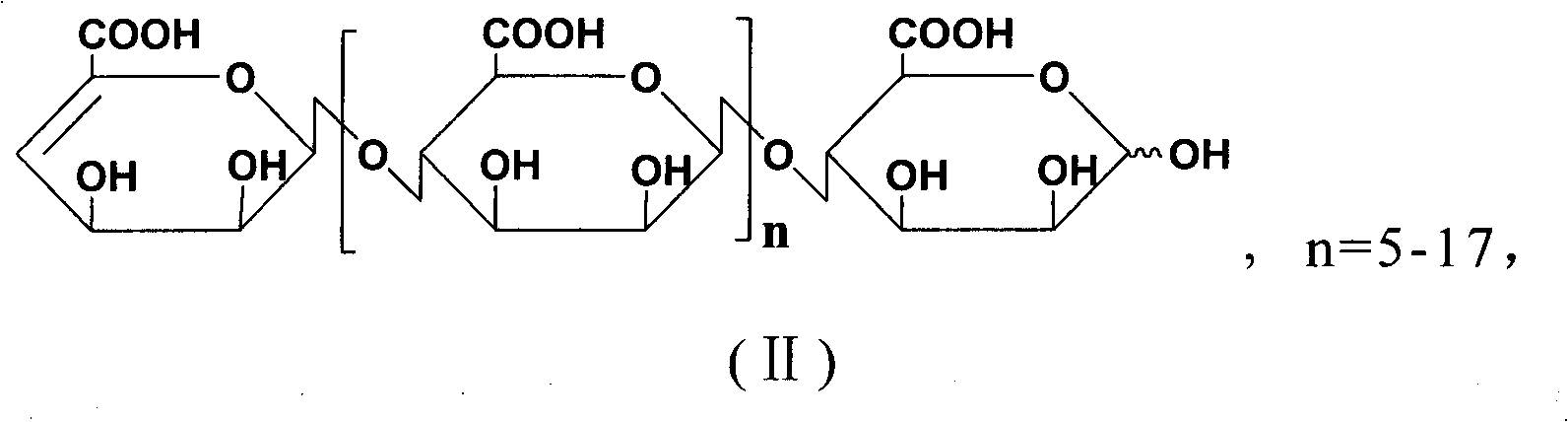
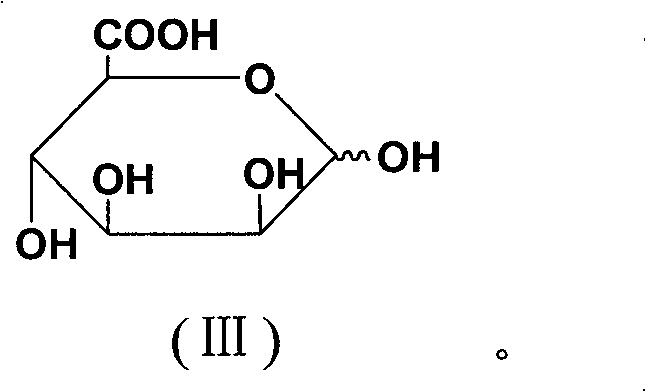
[0042]The basic raw material used in this process is alginic acid (extracted from kelp produced in the north, M / G is 7:3), and the molecule of alginic acid is β-D-mannuronic acid linked by (1→4) ( A linear block compound formed by linking M) units with (1→4) linked α-L-pyranguronic acid (G) units, and MG mixed segments.
[0043] 1. Preparation of oligomannuronic acid
[0044] A. Hydrolysis: Take 100g of food-grade alginate produced in the north, prepare a uniform glue solution with a concentration of 2% (W / V) with water, stir and reflux for 10 hours in hydrochloric acid solution of 0.5mol / L, and then separate by centrifuge , take the sediment;
[0045] B. Classification: Dissolve the above precipitate in 0.8% Na 2 CO 3 In the aqueous solution, adjust the pH value to 2.85 with 0.5mol / L hydrochloric acid, separate the solution, and take the supernatant;
[0046] C. Secondary hydrolysis: Ad...
Embodiment 2
[0056] Embodiment 2: Preparation of oligomeric mannuronic acid propyl ester sulfate sodium salt
[0057] The difference between this example and Example 1 is that in the above-mentioned step 2 of the esterification, HCl-methanol is replaced by propylene oxide, and sodium hydroxide is used as a catalyst. Put 50g of oligomeric mannuronic acid into a three-necked reaction bottle, add 200mL of propylene oxide, stir, then add 0.3g of NaOH, stir for 6 hours, wash the reaction product with 95% ethanol three times, and dry under reduced pressure to obtain manna Propyl uronic acid ester. All the other steps are the same as in Example 1 to prepare oligomeric mannuronic acid propyl ester sulfate sodium salt.
Embodiment 3
[0058] Embodiment 3: Drug efficacy test of antithrombotic drug for intravenous injection
[0059] The main pharmacological activity experimental results of the oligomeric mannuronate propyl sulfate sodium salt obtained by applying the method of the present invention are as follows, and the following results are obtained through systematic experimental research according to the method stipulated by the state.
[0060] A, the anticoagulant efficacy result of the antithrombotic drug for intravenous injection of the present invention
[0061] Table 1 The effect of the new drug on the coagulation index of rabbits (X±S, n=6)
[0062]
[0063] * : vs normal saline * p** p*** p<0.001
[0064] #: vs 6.25mg / kg new drug #p<0.05##p<0.01###p<0.001
[0065] : All greater than 120 seconds
[0066] The results showed that the new drug could significantly prolong TT, CT, APTT, RT, and PT of rabbits. Tip: This drug has obvious anticoagulant effect.
[0067] B, the influence of antithr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| esterification rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com