Caffeoyl derivative and use of coffeeoyl derivative in preparing drugs against respiratory syncytial viruses
An anti-respiratory and syncytial virus technology, applied in the field of medicine, can solve the problems of high cost, high toxicity and side effects, and no specific prevention and control measures, and achieve good application prospects and novel mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Grind the dried roots of Glycyrrhizae (collected from Guangdong and identified by Mai Zhenqiu, a senior engineer of Guangzhou Medicinal Materials Company), use 12 times the mass of Glycyrrhizae to percolate and extract with an ethanol aqueous solution with a concentration of 95% by mass, and extract the extract by rotating Concentrate under reduced pressure with an evaporator until there is no alcohol smell to obtain the total extract, suspend the total extract with 2 times the volume of distilled water, sequentially extract with petroleum ether, ethyl acetate and n-butanol, and recover the solvent under reduced pressure to obtain petroleum ether respectively Part, ethyl acetate part and n-butanol part; the n-butanol part is segmented by D101 macroporous adsorption resin column, and gradient elution is carried out with ethanol aqueous solution with a mass percentage concentration of 0-95%, and the collected mass percentage concentration is 20-95%. The eluate was eluted w...
Embodiment 2
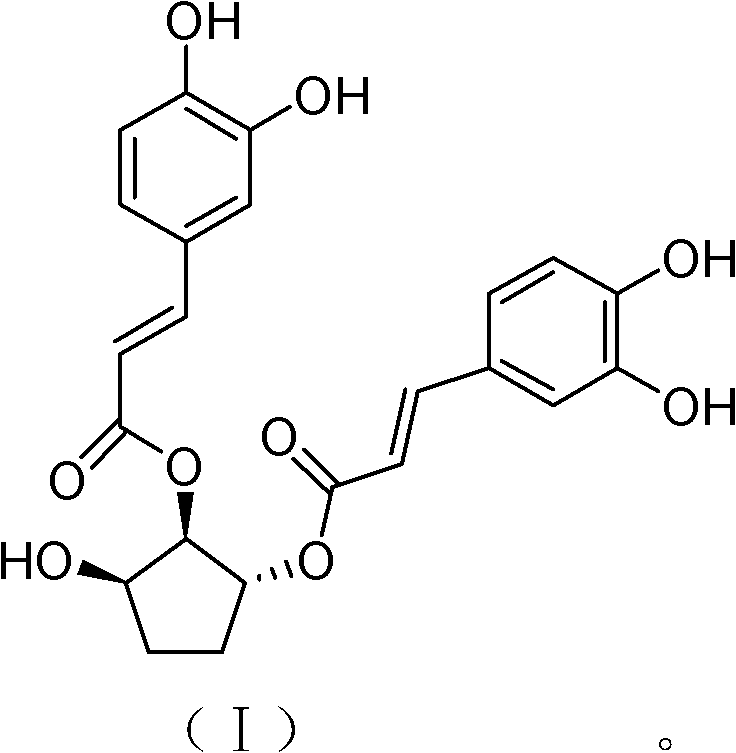
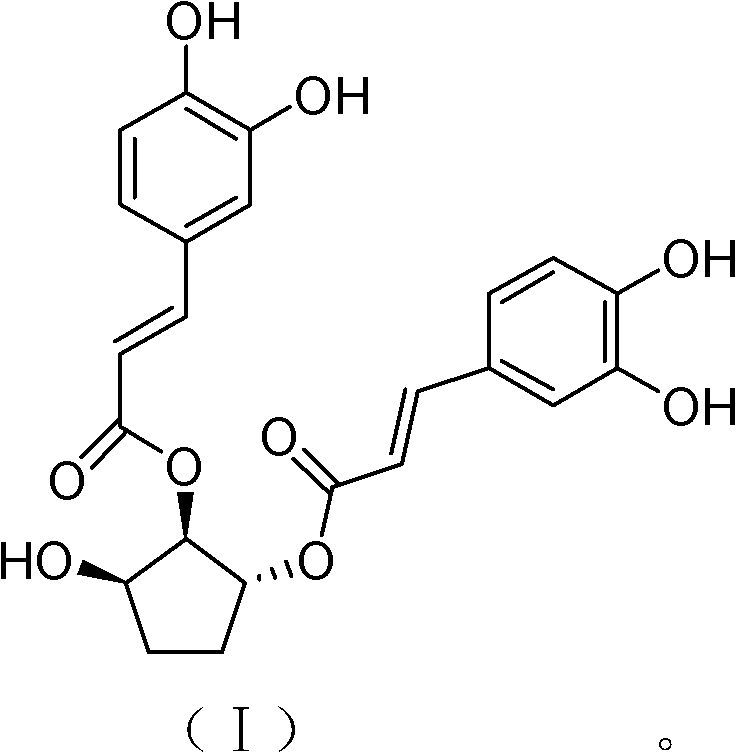
[0032] Example 2: In vitro anti-RSV activity of 1α, 2β-O-dicaffeoylcyclopent-3β-ol
[0033] (1) Cells, viruses and experimental materials: Respiratory syncytial virus (RSV, Long strain), host cells are laryngeal carcinoma cells (Hep-2 cells); positive control drug is ribavirin (ribavirin); cells grow at a mass percentage concentration 10% fetal bovine serum (FBS) in the MEM medium; the maintenance solution is the MEM medium containing 1% FBS in mass percent concentration.
[0034] (2) Preparation of sample solution: 1α obtained in Example 1, 2β-O-dicaffeoylcyclopent-3β-alcohol and ribavirin were prepared into solutions with a concentration of 40 mg / mL with dimethyl sulfoxide (DMSO), respectively. The samples were then prepared into 200 μg / mL and 10 μg / mL sample solutions with maintenance solution, which were used for cytotoxicity and antiviral activity experiments, respectively.
[0035] (3) measure the cytotoxicity of compound with tetrazolium salt (MTT) colorimetry:
[003...
Embodiment 3
[0043] Example 3: Determination of in vitro anti-RSV activity of 1α, 2β-O-dicaffeoylcyclopent-3β-ol combined with ribavirin
[0044] (1) Cells, viruses and experimental materials: Respiratory syncytial virus (RSV, Long strain), host cells are laryngeal carcinoma cells (Hep-2 cells); positive control drug is ribavirin (ribavirin); cells grow at a mass percentage concentration 10% fetal calf serum (FBS) MEM medium; the maintenance solution is 1% FBS MEM medium.
[0045] (2) Preparation of sample solution: 1α obtained in Example 1, 2β-O-dicaffeoylcyclopent-3β-ol sample and ribavirin sample were prepared with dimethyl sulfoxide (DMSO) to form a concentration of 40mg / mL 1α, 2β-O-dicaffeoylcyclopent-3β-ol and ribavirin were prepared into 0.31μg / mL and 0.62μg / mL solutions with maintenance solution, and then the two were mixed at a volume ratio of 1:1 into the sample solution to be tested.
[0046] (3) The cytopathic effect reduction assay was used to measure the antiviral activity ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap