Method for selecting a high expression recombinant cell line
一种细胞、选择性的技术,应用在重组DNA技术、生物化学设备和方法、使用载体引入外来遗传物质等方向,能够解决耗费时间和成本、劳动密集等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0065] Example 1: Introducing an inoperable polyA
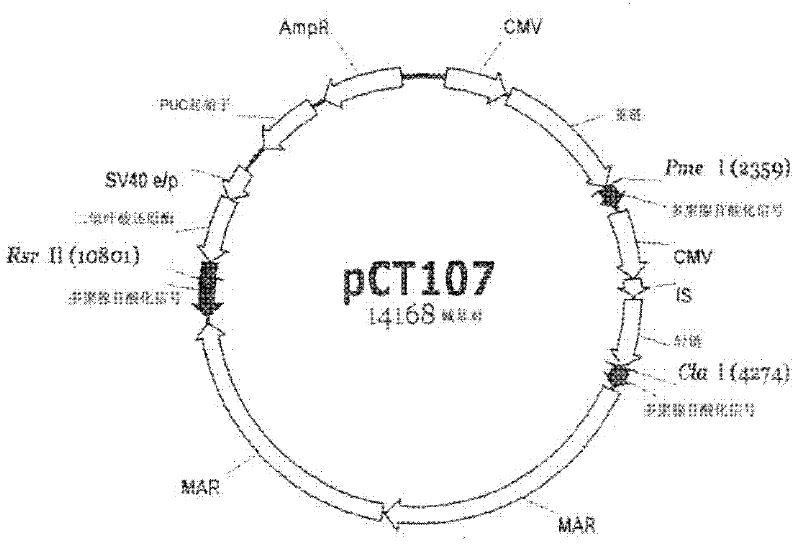
[0066] In the first step of examining gene expression levels based on the presence or absence of polyA, in order to be operably linked to the pCT107 vector expressing an IgG antibody ( figure 1 ), the polyA at the 3' end of the three transcription units (dhfr gene, heavy chain gene, and light chain gene) in ) was inoperable, and the pCT107 vector was cleaved with each of Rsr II, PmeI, and Cla I restriction enzymes. Restriction enzymes Rsr II, Pme I, and ClaI are specific sequences that exist between the 3' end of each of the dhfr gene, heavy chain gene, and light chain gene and the corresponding polyA to enable the expression of IgG antibodies. Linearized enzymes.
[0067] Treatment with restriction enzymes was performed in the following manner. Prepare three test tubes and add (i) 30 μg pCT107 vector DNA and 10 units (U) Rsr II (R0501S, NEB); (ii) 30 μg pCT107 vector DNA and 10 units Pme I (R0560S , NEB); and (iii) 30 m...
example 2
[0068] Example 2: Transfection into CHO cell lines
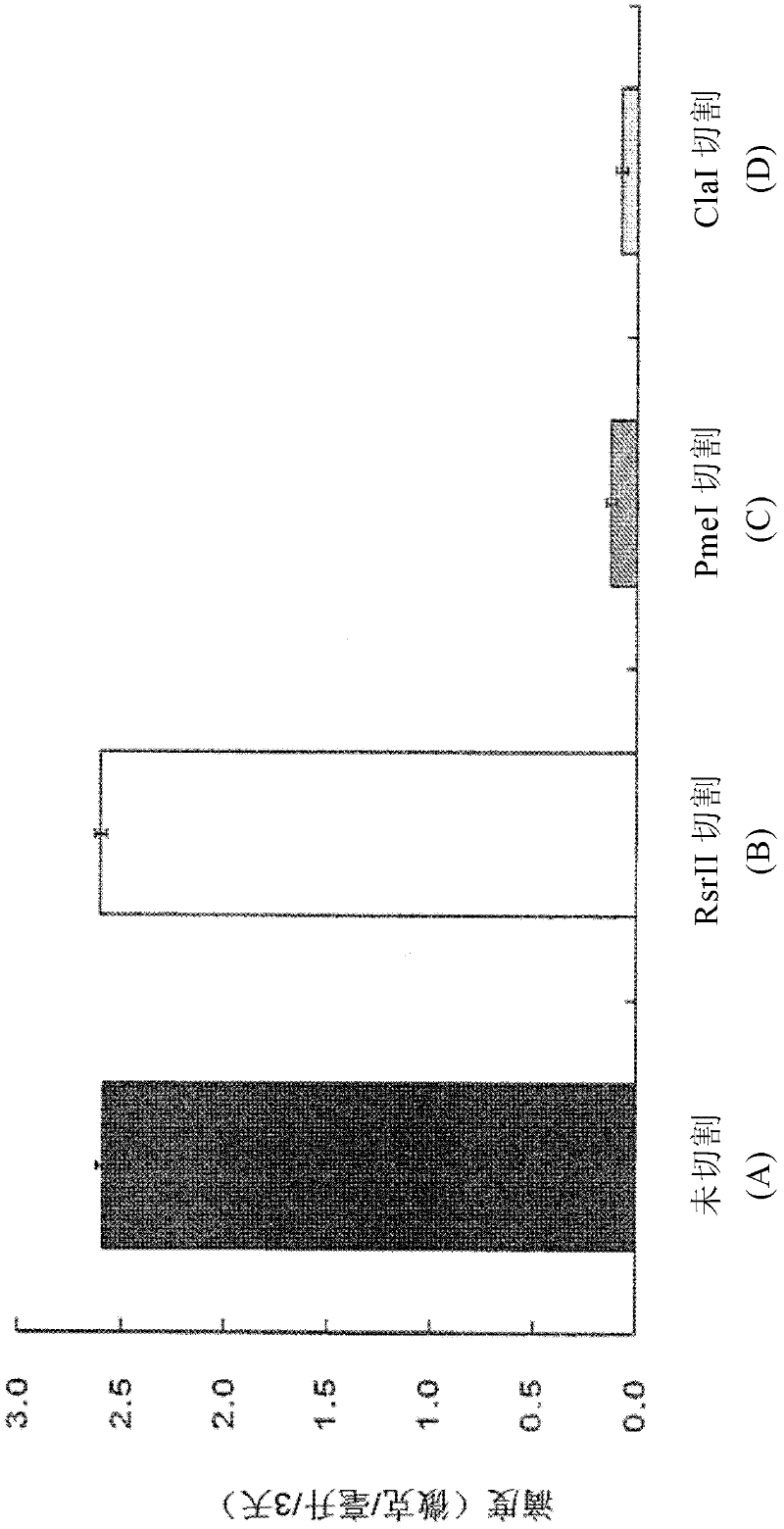
[0069] The vector linearized according to Example 1 was transfected into the same amount of CHO DG44 cells. Transfection in the control and test groups shown in Table 1 below was performed.
[0070] [Table 1]
[0071] Control group (A)
[0072] Transfection was performed as follows. Using MEMα medium (1140076, Invitrogen) supplemented with 10% FBS (12105, Sigma) to culture CHO DG44 cells at 0.5×10 6 Cells / well were seeded into 6-well plates, and after 24 hours, the medium was replaced with FBS-free MEMα medium. After 30 minutes, 2.5 μg of vector DNA from each of the control group (A), test group (B), test group (C) and test group (D) was added together with 500 μl of Opti-SFM medium (12309-050 , Invitrogen) were added together to each well, after which 6.25 microliters of LTX (15338-100, Invitrogen) were added thereto and vortexed well using a vortex mixer. The resulting mixture was allowed to stand at room ...
example 3
[0073] Example 3: Determination of IgG Antibody Gene Expression Using ELISA
[0074] In order to determine whether the IgG antibody gene was expressed, ELISA was performed 3 days after transfection. First, goat anti-human immunoglobulin G (Fcγ) (109-006-098, Jackson ImmunoReserarch) was adsorbed onto a 96-well microtiter plate (449824, Nunc). Plates were blocked with phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA, Bovine Serum Albumin), and serially diluted samples were added to each well. After allowing the plates to stand at room temperature for 2 hours, samples were treated with a peroxidase-labeled goat anti-human kappa antibody (I1514, Sigma) for detection. After standing at room temperature for 1 hour, the sample was reacted with tetramethyl benzidine (TMB, tetramethyl benzidine), and 1N HCl was added thereto to stop the reaction. Human IgG1κ (A7164, Sigma) purified from myeloma plasma at a concentration of 250 ng / ml was used as a standard. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com