Homogeneous agglutination immunoassay method and kit for such method
An immunoassay and reagent technology, applied in the field of homogeneous agglutination assay, can solve the problems of easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] 1) Reagent
[0073] The reagents used have the following composition:
[0074] i) Reagent A
[0075]
[0076] Join H 2 O up to 1000mL. Optionally, preservatives, stabilizers, EDTA or protein can be added to Reagent A. However, they are not required for the method according to the invention.
[0077] ii) Reagent B
[0078]
[0079] Join H 2 O up to 1000mL. Optionally, preservatives, stabilizers, EDTA, proteins, or detergents may be included in Reagent B. However, they are not required for the method according to the invention.
[0080] iii) Calibrator
[0081] A set of calibrators with a concentration range of 0-530mg / L CRP.
[0082] 2) Determination
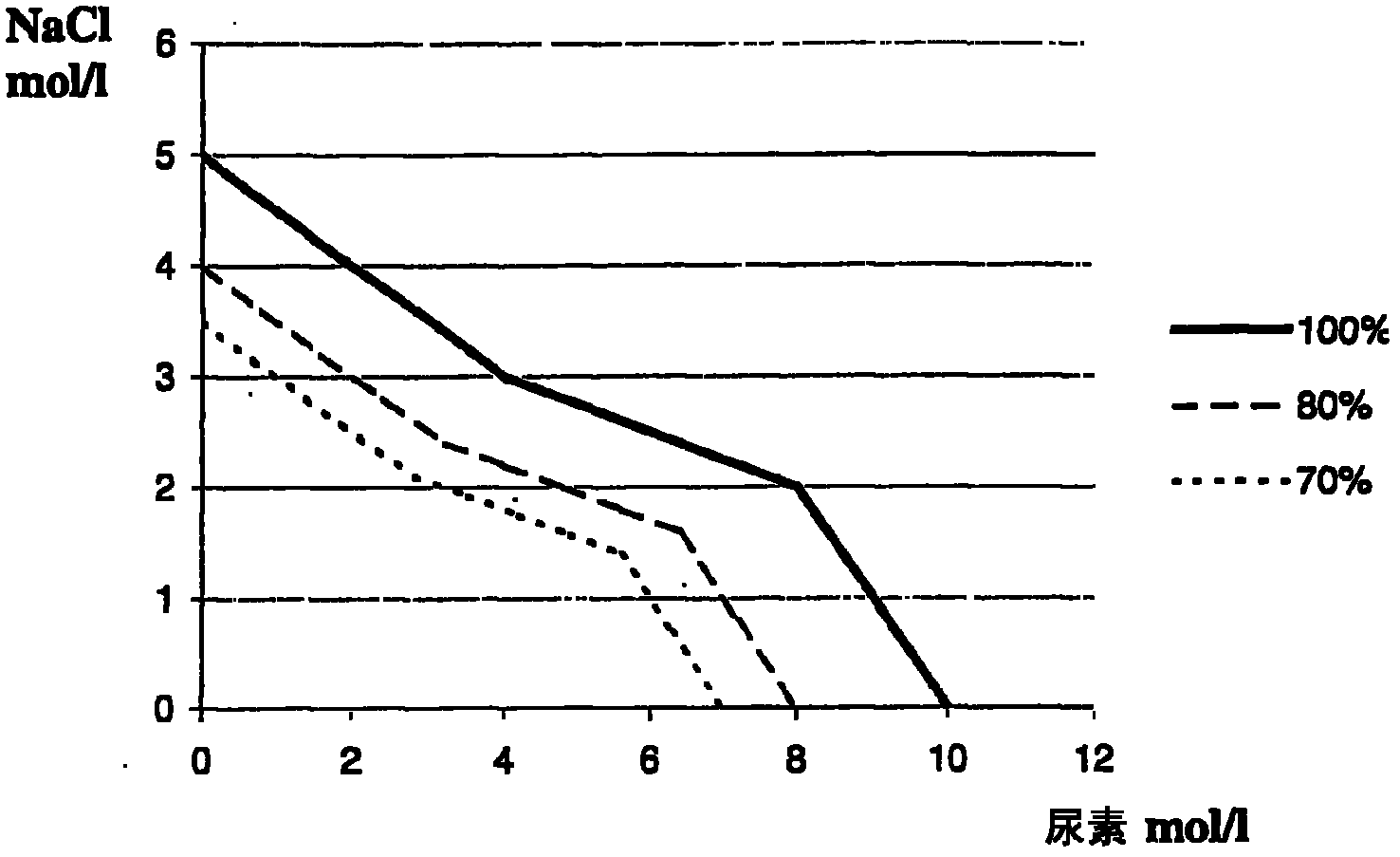
[0083] The determination is carried out at 37° C. in an automatic analyzer Olympus AU2700. All the different urea concentrations of reagent A were used in combination with each particle size of reagent B.
[0084] - 2 μL of Calibrator was pipetted into 150 μL of Reagent A and mixed;
[0085] - After 3.2 m...
Embodiment 2
[0091] 1) Reagent
[0092] Select reagent A with a urea concentration of 8.0 mol / L. As in the case of Example 1, Reagent B can contain either 158 nm diameter particles or 101 nm diameter particles.
[0093] 2) Determination
[0094] Similar to Example 1, the entire assay was performed in an Olympus AU2700 analyzer at 37°C.
[0095] - 2 µL of each calibrator was pipetted into 150 µL of Reagent A and mixed;
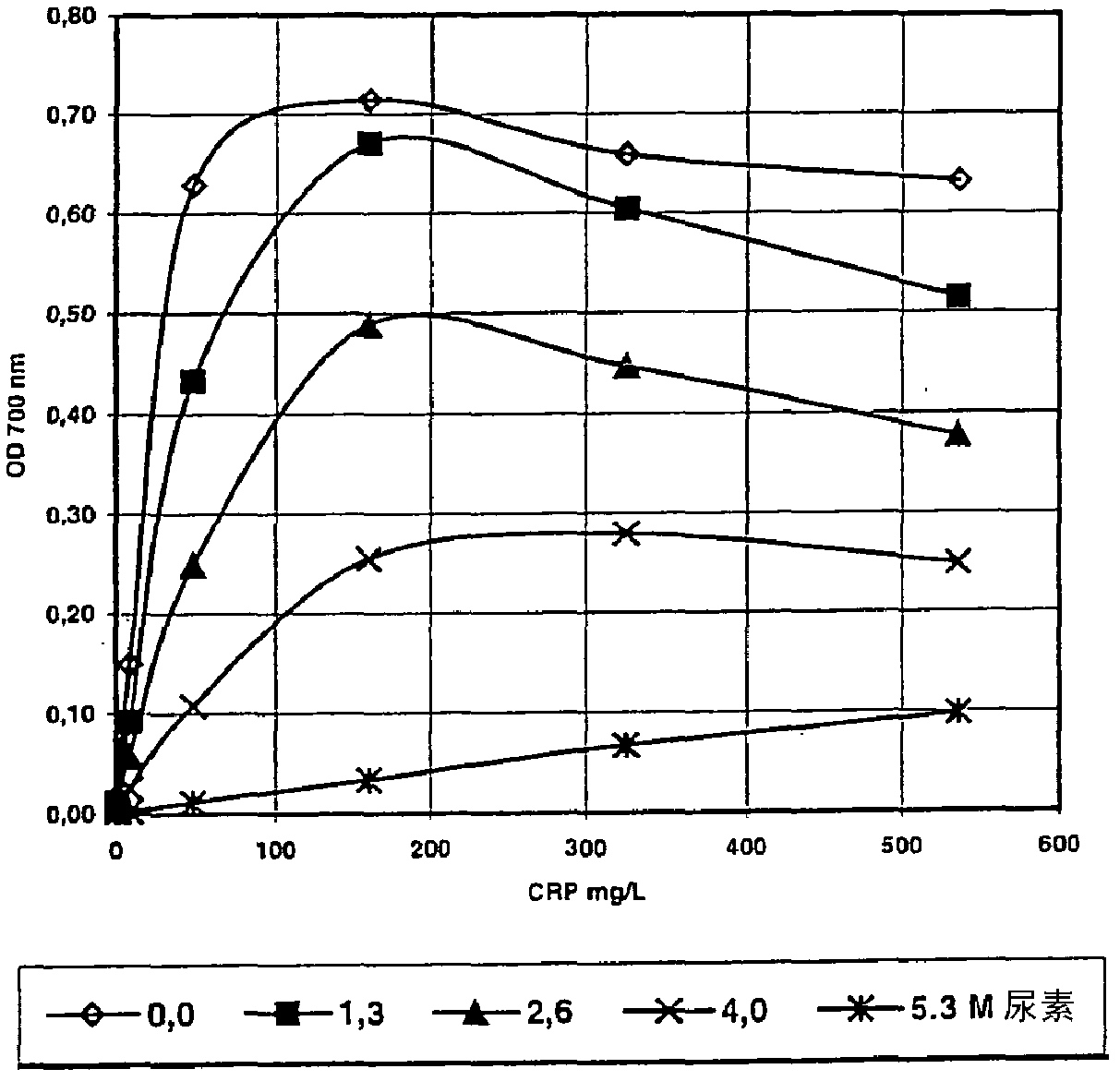
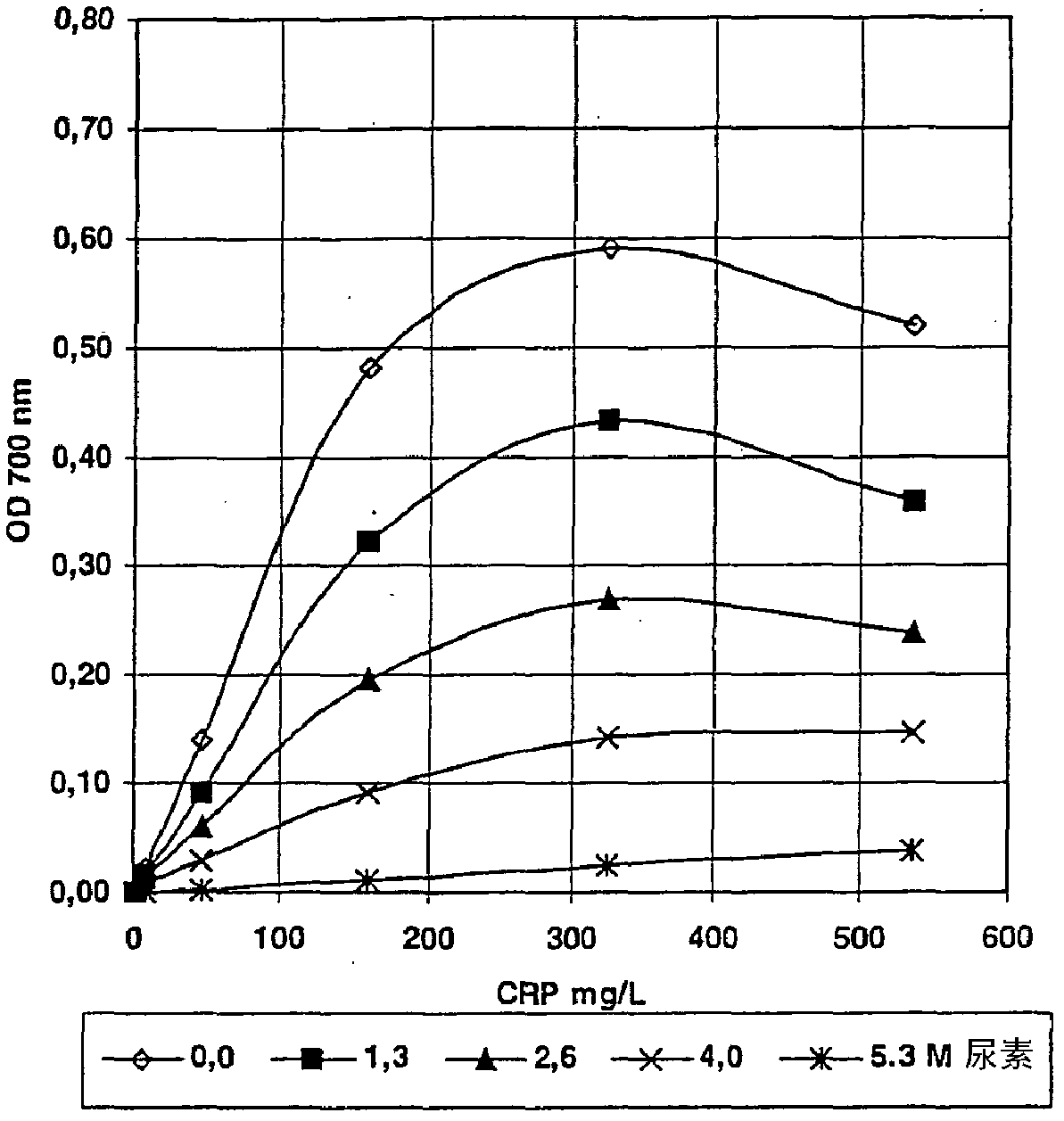
[0096] - After 3.2 min, add 75 μL of Reagent B and mix.
[0097] - Then measure the OD value at 520nm, 600nm or 700nm during 4.9 minutes.
[0098] - For each wavelength, the change in OD value is expressed as the change between the beginning and end of the measurement period.
[0099] -For each wavelength, the change in OD is plotted against the concentration of CRP in a single calibrator ( Figure 4 (158nm); Figure 5 (101nm)).
[0100] figure 2 and image 3 The measurement results of Example 1 using 2 different particle sizes at different urea concentrations ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com