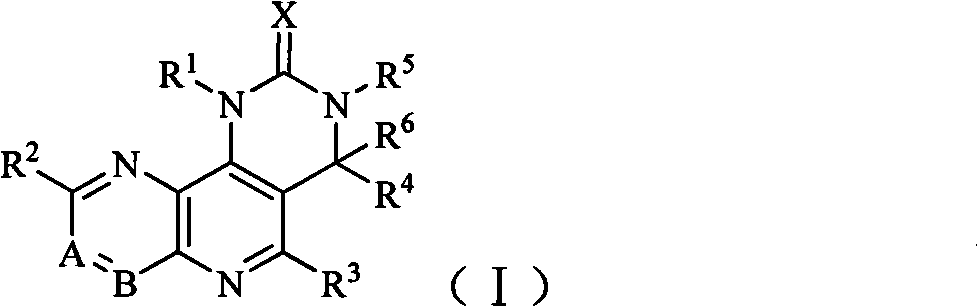
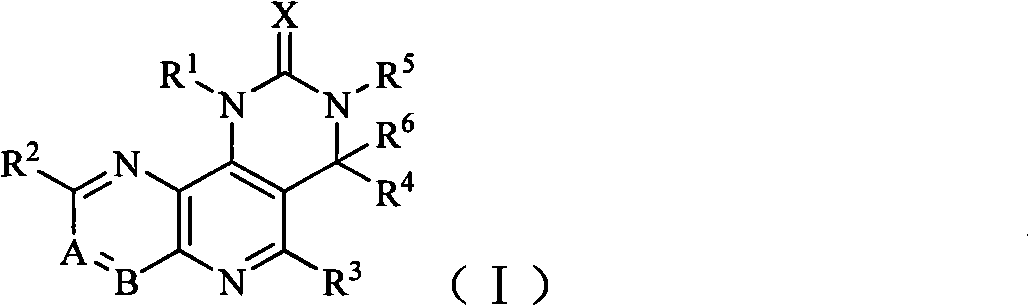Tricyclic dual PI3K and mTOR inhibitors
A cycloalkyl and heterocyclyl technology, applied in the field of tricyclic PI3K and mTOR dual inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
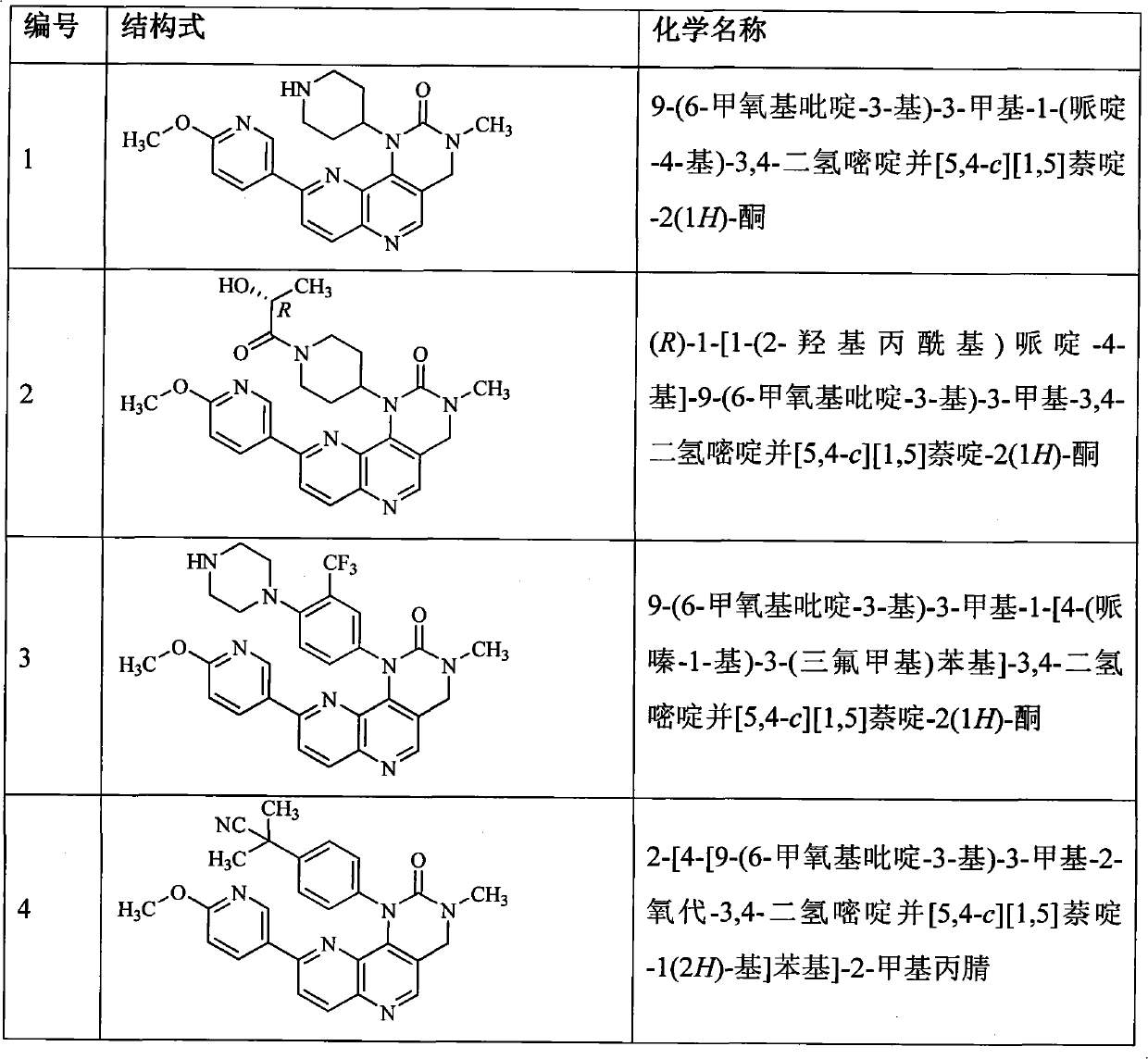
[0148] Example 1 9(6-methoxypyridine 3-yl)-3-methyl-1-(piperidin-4-yl)-3,4-dihydropyrimido[5,4-c][1, 5] Naphthyridine Preparation of -2(1H)-one (compound 1)
[0149]
[0150] (1) Preparation of 4-chloro-6-(6-methoxypyridine 3-yl)-1,5-naphthyridine 3-carboxylate
[0151]
[0152] Ethyl 4,6-dichloro-1,5-naphthyridine-3-carboxylate (0.472g, 1.74mmol), 6-(methoxy)pyridin-3ylboronic acid (0.535g, 3.5mmol), Pd (PPh 3 ) 2 Cl 2 (0.119g, 0.17mmol) and K 2 CO 3 (0.718g, 5.2mmol) was added to dioxane (10mL) and water (5mL) in sequence, the nitrogen was replaced, the tube was sealed at 90°C for 4h, quenched with water, extracted with dichloromethane, dried, and column chromatography, 0.373 g of the product was obtained, and the yield was 62.4%.
[0153] (2) 4-[1-(tert-butoxycarbonyl)piperidin-4-ylamino]-6-(6-methoxypyridin-3-yl)-1,5-naphthyridine-3-carboxylic acid ethyl Preparation of esters
[0154]
[0155] 4-Aminopiperidine-1-carboxylic acid tert-butyl ester (2.0 g...
Embodiment 2
[0169] Example 2 (R)-1-[1-(2-hydroxypropionyl)piperidin-4-yl]-9-(6-methoxypyridin-3-yl)-3-methyl-3,4 -dihydropyrimide Preparation of pyrido[5,4-c][1,5]naphthyridin-2(1H)-one (compound 2)
[0170]
[0171] Dissolve R-lactic acid (133 mg, 1.2 mmol) in 7 mL of DMF, add 2-(7-azobenzotriazole)-N, N, N', N'-tetramethyluronium hexafluorophosphoric acid at room temperature Ester (HATU) (456 mg, 1.2 mmol), 9-(6-methoxypyridin-3-yl)-3-methyl-1-(piperidin-4-yl)-3,4-dihydropyrimidino [5,4-c][1,5]Naphthyridin-2(1H)-one (compound 1) (405mg, 1.0mmol), stirred overnight at room temperature, poured into 5mL water, precipitated solid, filtered, dried to obtain the product 223 mg, yield 46.8%.
[0172] Molecular formula: C 25 h 28 N 6 o 4 Molecular weight: 476.53 Mass spectrum (M+H): 477
Embodiment 3
[0173] Example 3 9-(6-methoxypyridin-3-yl)-3-methyl-1-[4-(piperazin-1-yl)-3-(trifluoromethyl)phenyl]-3 , 4-dihydropyrimide Preparation of pyrido[5,4-c][1,5]naphthyridin-2(1H)-one (compound 3)
[0174]
[0175] (1) 4-[4-[4-(tert-butoxycarbonyl)piperazin-1-yl]-3-(trifluoromethyl)phenylamino]-6-(6-methoxypyridine-3- base)-1,5-naphthyridine-3-carboxylate ethyl ester
[0176]
[0177] Refer to (2) in Example 1 for specific operations, throw 4-[4-amino-2-(trifluoromethyl)phenyl]piperazine-1-carboxylic acid tert-butyl ester (3.799g, 11mmol), 4- Ethyl chloro-6-(6-methoxypyridin-3-yl)-1,5-naphthyridine-3-carboxylate (2.5g, 7.27mmol) was used to obtain 3.31g of the product with a yield of 69.8%.
[0178] (2) 4-[4-[3-Formyl-6-(6-methoxypyridin-3-yl)-1,5-naphthyridin-4-ylamino]-2-(trifluoromethyl) Preparation of tert-butyl phenyl]piperazine-1-carboxylate
[0179]
[0180] The specific operation refers to (3) in Example 1, cast 4-[4-[4-(tert-butoxycarbonyl)piperazin-1-yl]-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com