Borate luminescent glass and preparation method thereof
A technology of luminescent glass and borate, applied in the field of luminescent materials, can solve the problem that the phosphor powder cannot meet the research and practical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
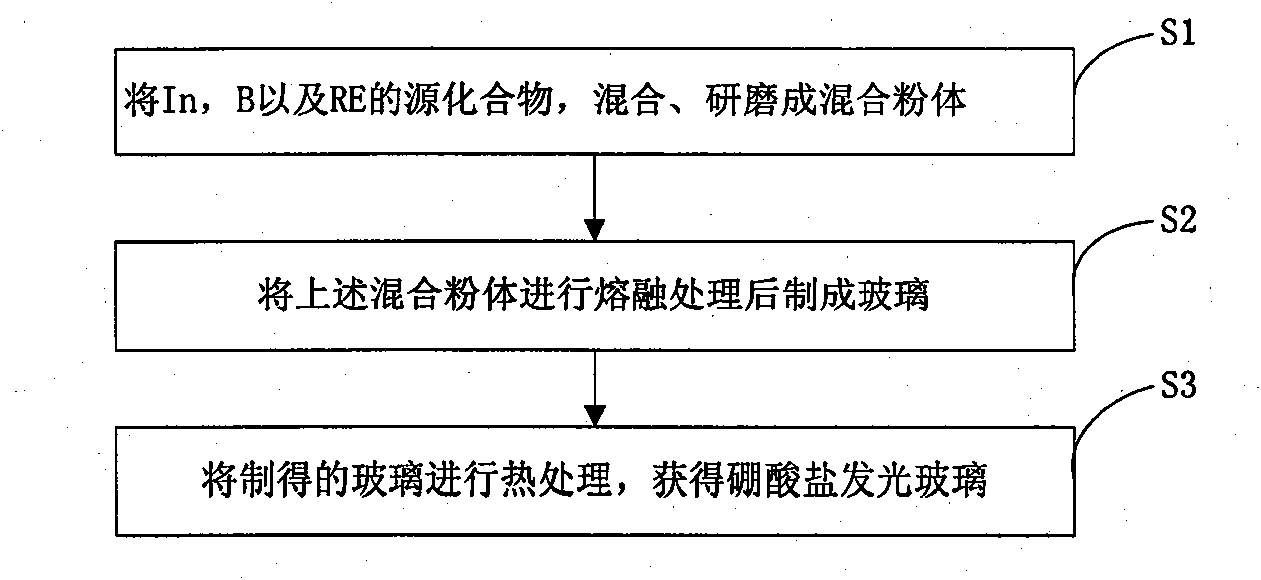
[0019] The preparation method of above-mentioned a kind of borate luminous glass, such as figure 1 As shown, the preparation process is as follows:
[0020] Step S1, according to the general chemical formula (50-x) In 2 o 3 -50B 2 o 3 -xRE 2 o 3 The molar number of each component in the formula, weigh the source compound of In, the source compound of B and the source compound of RE, mix and grind them into a mixed powder; wherein, the value of x is 0<x≤10, and RE is Eu ion or at least one of Tb ions;
[0021] Step S2, melting the mixed powder at a temperature of 1450-1600°C for 1-5 hours, taking it out and pouring it on a steel plate for rapid cooling to form glass;
[0022] Step S3, heat-treating the above-obtained glass at 500-900°C for 1-20 hours, and then cooling to prepare the borate luminescent glass.
[0023] In the above step S1, preferably, the source compound of In, the source compound of B and the source compound of RE are respectively In 2 o 3 、H 3 BO 3...
Embodiment 1
[0026] Weigh chemically pure In with an analytical balance 2 o 3 14.08g, H 3 BO 3 6.96g and Eu 2 o 3 1.98g, the raw materials were placed in a mortar and mixed thoroughly, and the mixed raw materials were poured into a corundum crucible. The raw material is melted in a high-temperature furnace at 1550°C for 3 hours, then taken out and poured onto a steel plate for rapid cooling to obtain a composition of 45In 2 o 3 -50B 2 o 3 -5Eu 2 o 3 borate glass. The obtained glass was kept at 500° C. for 5 h and then cooled in a furnace to obtain Eu ion-doped borate luminescent glass, which produced strong red emission under excitation of cathode ray and blue light.
[0027] figure 2 It is the photoluminescence spectrum of the luminescent glass obtained in Example 1. In the figure, Ex1 is the excitation spectrum with the excitation peak wavelength at 465 nm, and Em1 is the emission spectrum with the emission peak wavelength at 612 nm.
Embodiment 2
[0030] Weigh chemically pure In with an analytical balance 2 o 3 14.03g, H 3 BO 3 6.94g and Tb 4 o 7 2.09g, the raw materials were placed in a mortar and mixed thoroughly, and the mixed raw materials were poured into a corundum crucible. The raw material is melted in a high-temperature furnace at 1550°C for 5 hours, then taken out and poured onto a steel plate for rapid cooling to obtain a composition of 45In 2 o 3 -50B 2 o 3 -5Tb 2 o 3 borate glass. The obtained glass was kept at 700° C. for 5 hours and then cooled in a furnace to obtain a borate luminescent glass doped with Tb ions. The luminescent glass produced strong green light emission under the excitation of cathode ray and blue light.
[0031] Figure 4 It is the photoluminescence spectrum of the luminescent glass obtained in Example 2, Ex2 in the figure is the excitation spectrum, the excitation peak wavelength position is located at 485nm, Em2 is the emission spectrum, and the emission peak wavelength po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com