Vangl1 peptides and vaccines including the same
An immunology and antigen technology, applied in the field of drugs for the treatment and prevention of tumors, can solve problems such as low objective response rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0352] Materials and methods
[0353] cell line
[0354] The A24 lymphoblastoid cell line (A24LCL) was established by transforming Epstein-bar virus into HLA-A24 positive human B lymphocytes. COS7, an African green monkey kidney cell line, was purchased from ATCC.
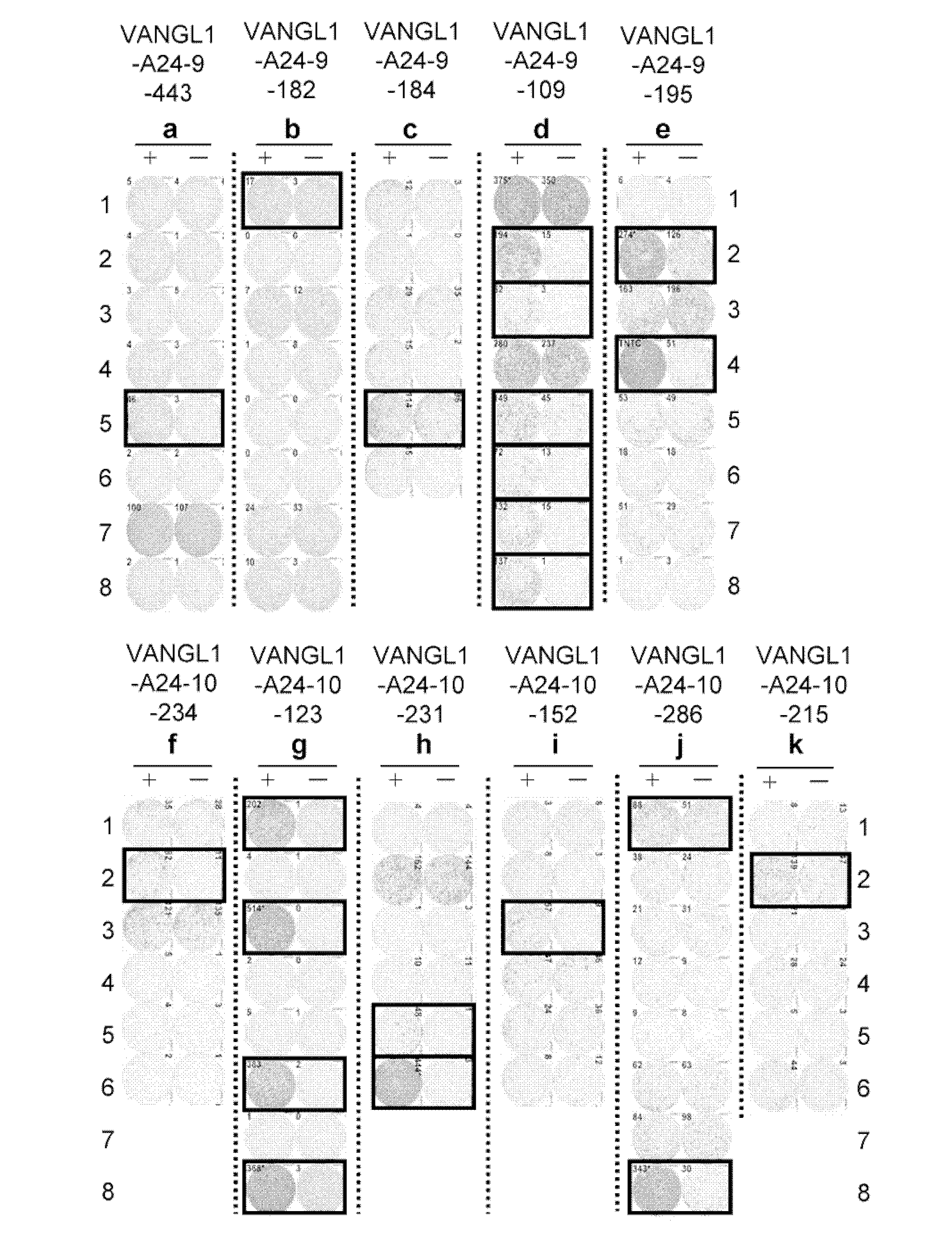
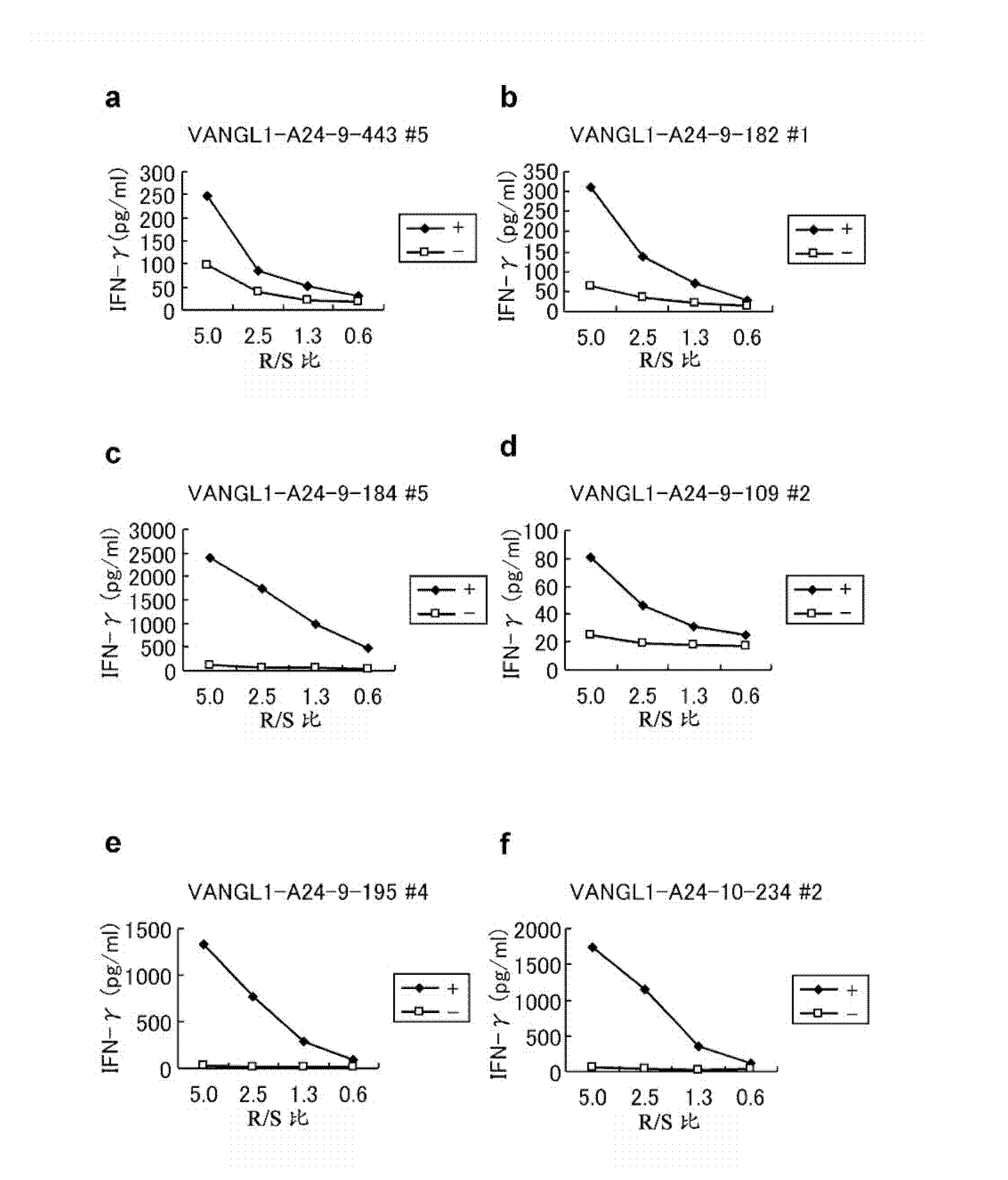
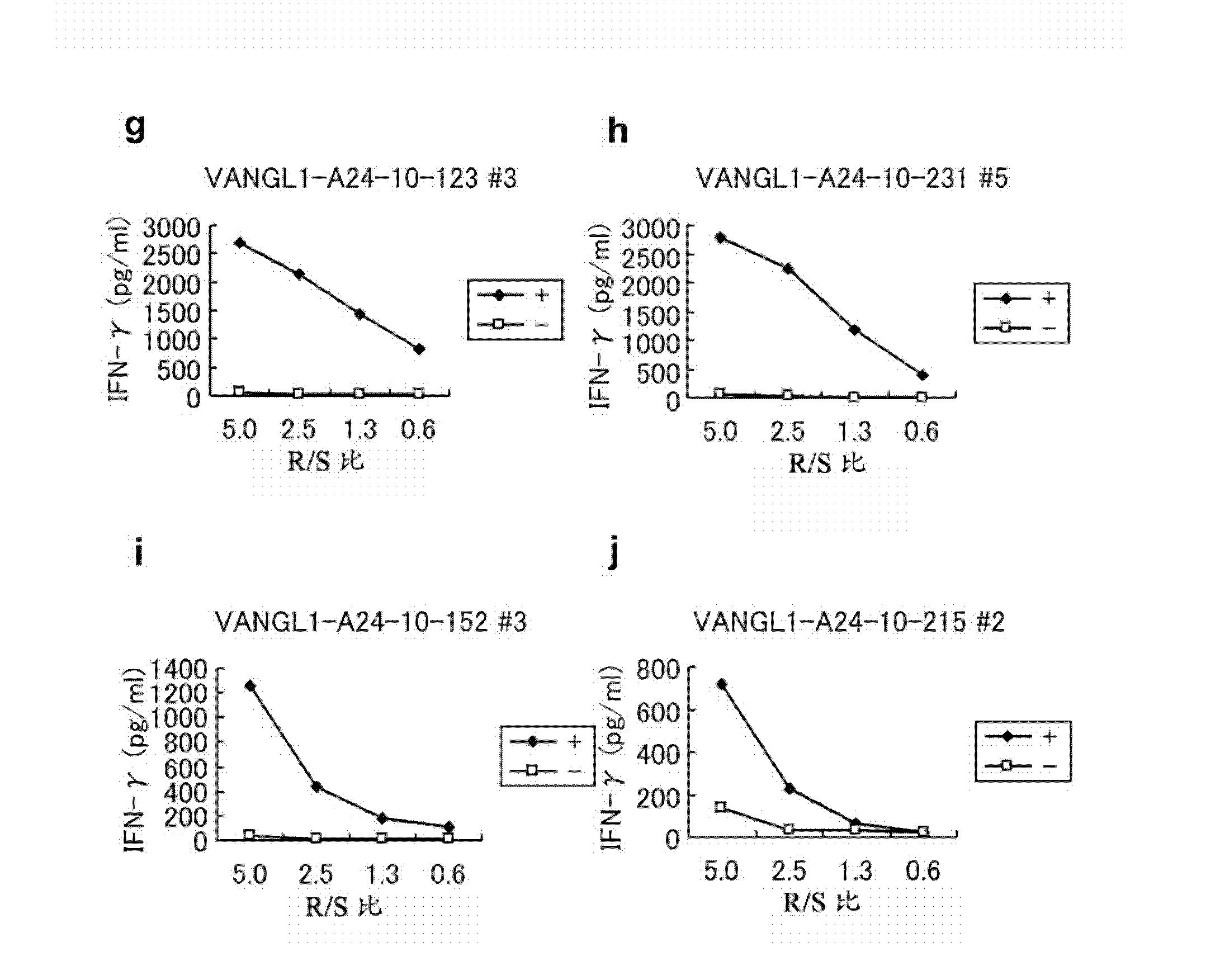
[0355] Selection of candidates for VANGL1-derived peptides
[0356] Using the binding prediction software "BIMAS" (www-bimas.cit.nih.gov / molbio / hla_bind) (Parker et al. (J Immunol 1994, 152(1): 163-75), Kuzushima et al. (Blood 2001, 98(6):1872-81))) predicts VANGL1-derived 9-mer and 10-mer peptides that bind HLA-A*2402 molecules. The peptides were synthesized by SIGMA (Sapporo, Japan) following standard solid phase synthesis and purified by reverse phase high performance liquid chromatography (HPLC). The purity (>90%) and identity of the peptides were determined by analytical HPLC and mass spectrometry analysis, respectively. Peptides were dissolved in dimethyl sulfoxide (DMSO) at 20 mg / ml and stored at -8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com