Pharmaceutical Composition For Treating And Preventing Diseases Involving Joints And Connective Tissue
A technology of connective tissue and composition, applied in the direction of drug combination, bone disease, active ingredients of hydroxyl compounds, etc., can solve problems such as long recovery time, cost burden, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Preparation of Therapeutic Compositions
[0101] The therapeutic compositions shown in Table 1 below were prepared. The components shown in Table 1 were all purchased from Sigma Chemical Co. (USA).
[0102] [Table 1]
[0103]
Embodiment 2
[0104] Embodiment 2: Construction of test animal model
[0105] In this experiment, a rabbit model with a similar structure to the human knee joint was used. Specifically, 11 New Zealand White rabbits (average weight: 3.0±0.01 kg (2.8-2.9 kg), 8-month-old) were used. The reason for using mature rabbits is that osteoarthritis in rabbits is induced under similar conditions to humans, and the ability to regenerate cartilage is lower than that of immature rabbits.
[0106] Eleven rabbits were generally anesthetized and the knee joint of the right hind leg of the rabbit was subjected to cruciate ligament resection, after which the rabbit was left undisturbed for 6 weeks, thereby inducing arthritis in the rabbit.
[0107] Among these test rabbits, 6 rabbits were used as a test group. Six weeks after cruciate ligament resection, the test rabbits were general anesthetized, and then the therapeutic composition of Formulation 2 prepared in Example 1 was injected into the joint cavit...
Embodiment 3
[0110] Example 3: Determination of regeneration and repair of cartilage tissue
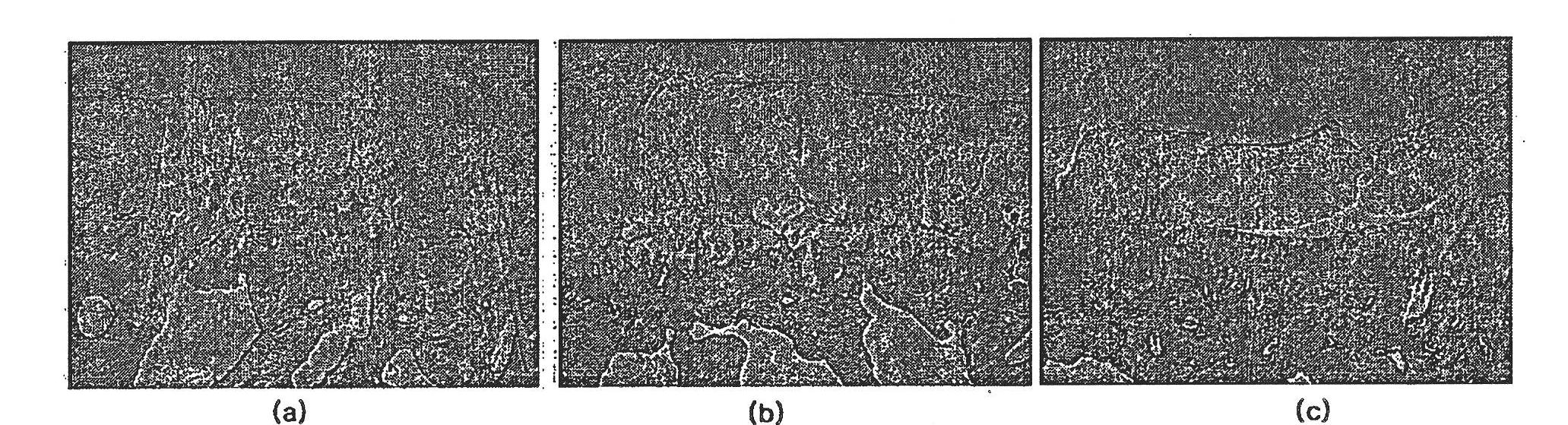
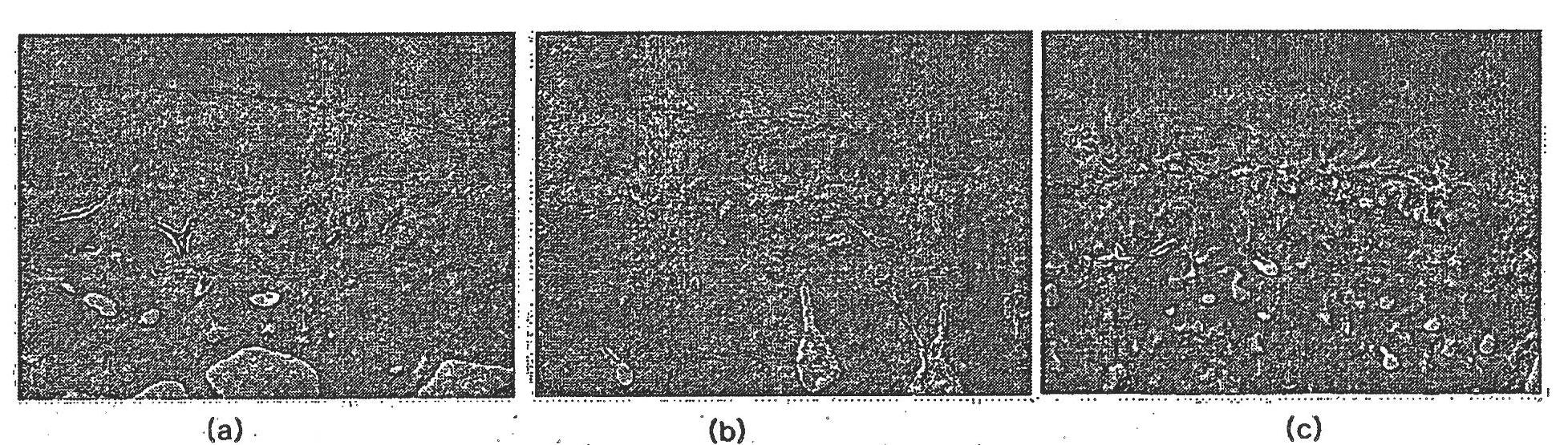
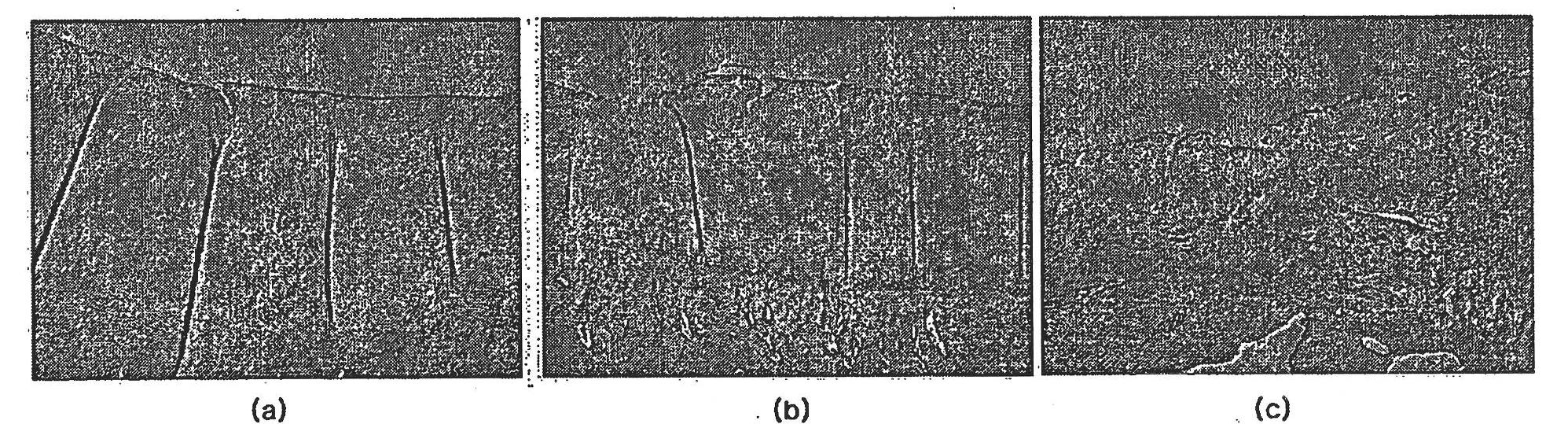
[0111] Four weeks after the last injection of the therapeutic composition of Formulation 2 of Example 1, the test rabbits were generally anesthetized and euthanized, and the left and right knee joints were harvested, immersed and fixed in 10% neutral formalin (pH 7.4) 24 hours or more.
[0112] The collected left and right knee joints of the test group and the control group were evaluated by histopathological evaluation method (H&E staining, modified Mankin's scoring method), and the degrees of articular cartilage damage and regeneration were compared between the test group and the control group.
[0113] Also, in order to evaluate the state of the normal left knee articular cartilage (normal group), the state of the right knee cartilage of the test group and the control group was corrected, and then the difference between the groups was statistically evaluated.
[0114] Figures 1 to 4 Shown ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap