Method for treating alkali wastewater containing uranium
A waste water treatment, alkaline technology, applied in the removal of radium, uranium in the alkaline uranium-containing wastewater of uranium mines, can solve the problems of poor radium removal effect, difficulty in reaching the maximum allowable limit of waste water discharge, etc., to achieve easy disposal, mining area The effect of environmental quality improvement and environmental pollution containment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Composition U of alkaline wastewater from a uranium mine: 3.91mg·L -1 ;CO 3 2- : 4.54g·L -1 ; HCO 3 - : 2.32g·L -1 ; Ra: 18.2Bq L -1 ;Cl - : 7.54g·L -1 ; Ca: 0.006g·L -1 ; Mg: 0.034g L -1 .
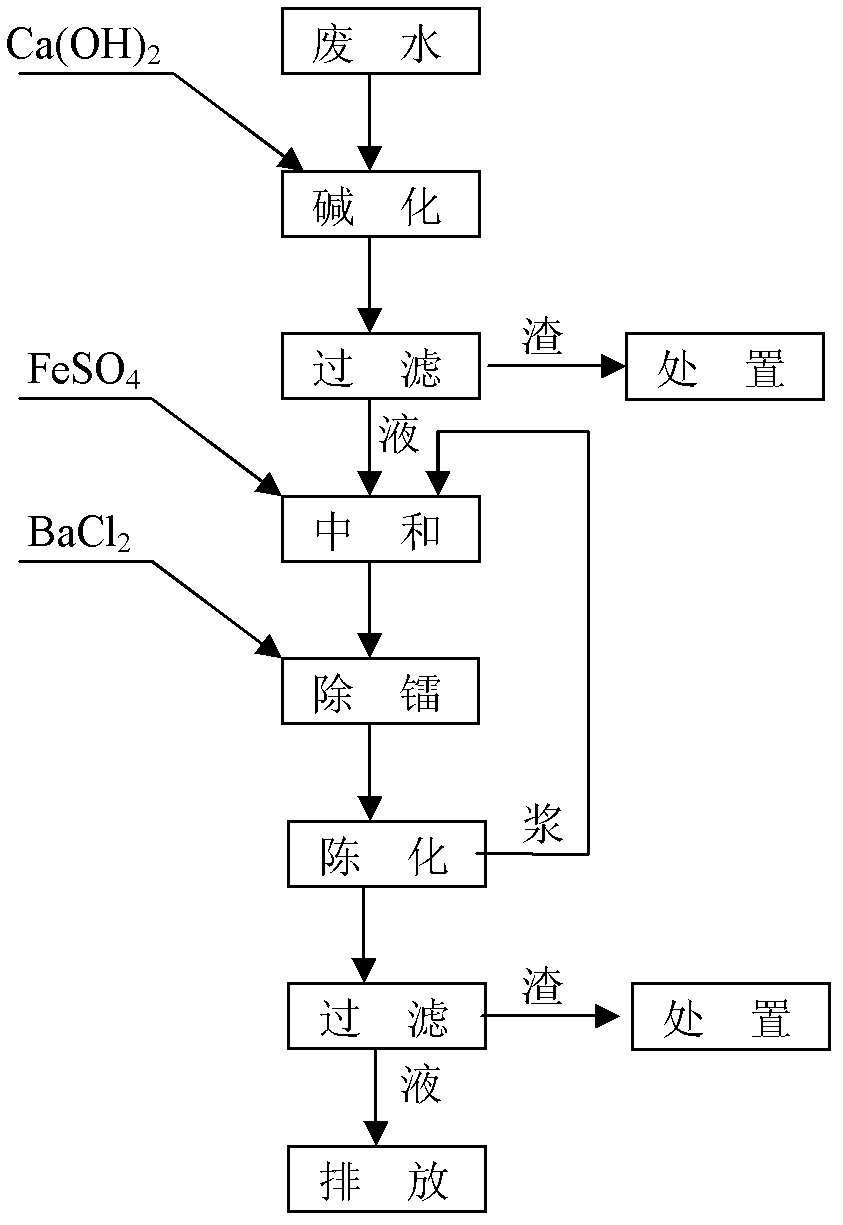
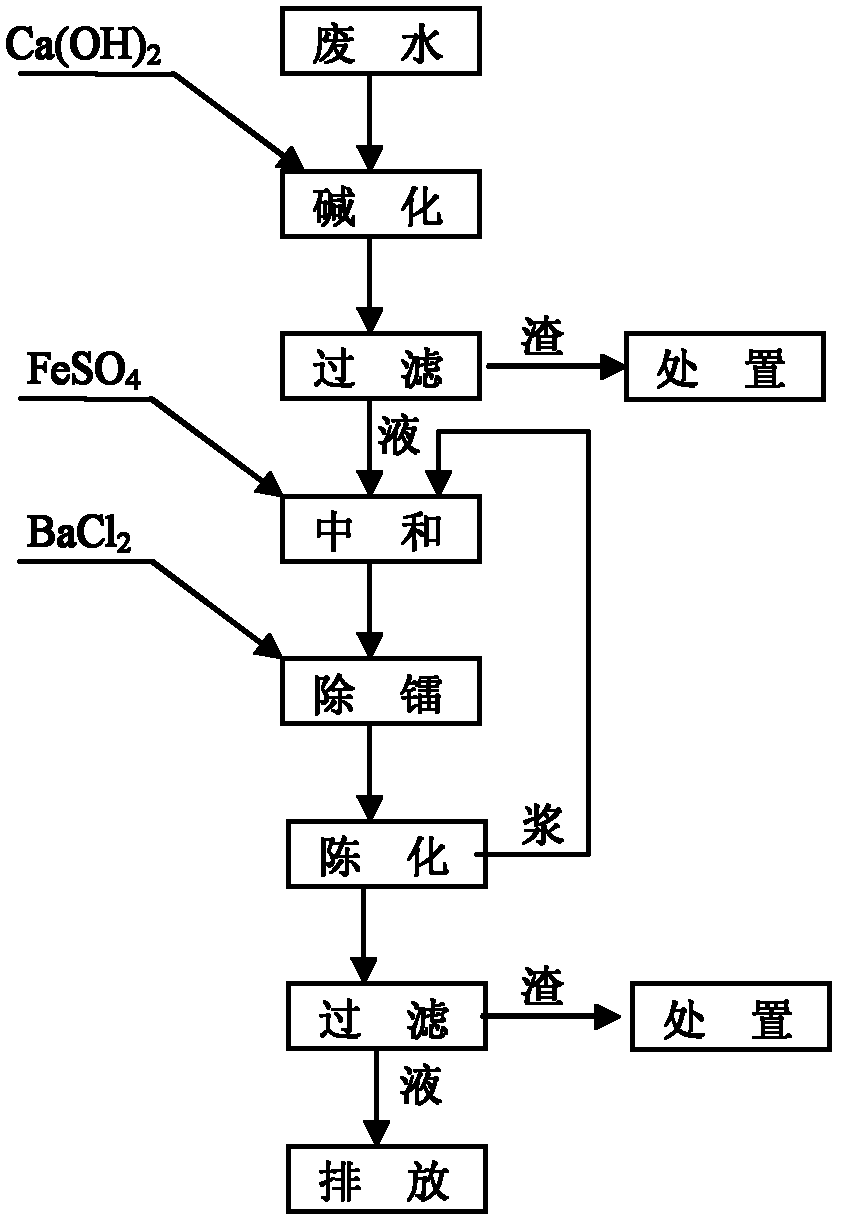
[0022] The above-mentioned alkaline uranium-containing wastewater treatment method comprises the steps of:
[0023] (a) Alkalization:
[0024] Adding Ca(OH) to alkaline uranium-containing wastewater 2 , the Ca(OH) 2 Made by digesting quicklime with water. Ca(OH) 2 with CO 3 2- and HCO 3 - reaction to generate CaCO 3 precipitation. Ca(OH) 2 The molar amount added is the amount of CO in alkaline uranium-containing wastewater 3 2- and HCO 3 - 1.1 times of the sum of moles, the reaction temperature is 25°C, and the reaction time is 1 hour. Ca(OH) 2 The amount added makes the CO in the alkaline uranium-containing wastewater 3 2- and HCO 3 - Concentration drops below 0.1mg / L;
[0025] (b) Neutralize:
[0026] Filtrating the alkaline uranium-containing wa...
Embodiment 2
[0033] Composition U of alkaline wastewater from a uranium mine: 0.70mg·L -1 ;CO 3 2- : 5.10g·L -1 ; HCO 3 - : 1.56g·L -1 ; Ra: 15.2Bq L -1 ;Cl - : 3.92g·L -1 ; Ca: 0.010g·L -1 ; Mg: 0.042g L -1 .
[0034] The above-mentioned alkaline uranium-containing wastewater treatment method comprises the steps of:
[0035] (a) Alkalization:
[0036] Adding Ca(OH) to alkaline uranium-containing wastewater 2 , the Ca(OH) 2 Made by digesting quicklime with water. Ca(OH) 2 with CO 3 2- and HCO 3 - reaction to generate CaCO 3 Precipitation, Ca(OH) 2 The amount added makes the CO in the alkaline uranium-containing wastewater 3 2- and HCO 3 - Concentration drops below 0.1mg / L;
[0037] Ca(OH) 2 The molar amount added is the amount of CO in alkaline uranium-containing wastewater 3 2- and HCO 3 - 1.1 times of the sum of moles, the reaction temperature is 25°C, and the reaction time is 1 hour.
[0038] (b) Neutralize:
[0039] Filtrating the alkaline uranium-conta...
Embodiment 3
[0046] Composition U of alkaline wastewater from a uranium mine: 9.49mg·L -1 ;CO 3 2- : 11.6g·L -1 ; Ra: 34.5Bq L -1 ;Cl - : 16.24g·L -1 ;Ca-1 ;Mg-1 .
[0047] The above-mentioned alkaline uranium-containing wastewater treatment method comprises the steps of:
[0048] (a) Alkalization:
[0049] Adding Ca(OH) to alkaline uranium-containing wastewater 2 , Ca(OH) 2 with CO 3 2- and HCO 3 - reaction to generate CaCO 3 Precipitation, Ca(OH) 2 The amount added makes the CO in the alkaline uranium-containing wastewater 3 2- and HCO 3 - Concentration drops below 0.1mg / L;
[0050] Ca(OH) 2 The molar amount added is the amount of CO in alkaline uranium-containing wastewater 3 2- and HCO 3 - 1.1 times of the sum of moles, the reaction temperature is 25°C, and the reaction time is 1 hour.
[0051] (b) Neutralize:
[0052] Filtrating the alkaline uranium-containing wastewater obtained in step (a) to obtain a filtrate and a filter residue; then adding FeSO to the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com