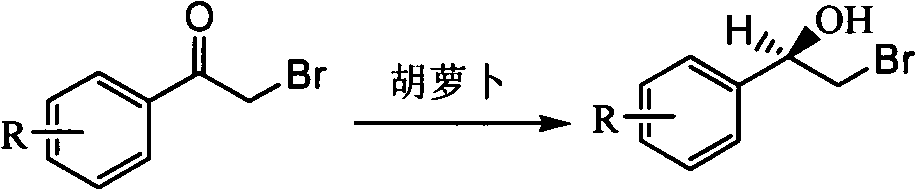
Method for catalytic synthesis of R-2-bromo-1-aryl alcohol using carrot root whole cells
A technology of R-2-, aryl ethanol is applied in the field of preparing optically active alcohol by biocatalysis method, and the effect of high application value is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: add 0.1mol / L, the phosphate buffer saline solution 50mL of pH=7.0 and the chopped carrot root cell of 10g in an Erlenmeyer flask, vibrate (120r / min ) for 20 min to activate carrot root cells, then add 0.02 g (0.4 g / L) α-bromoacetophenone, and shake and react at the same temperature for 24 h. After the reaction, 40mL of ethyl acetate was added to the reaction solution, centrifuged after shaking and extraction, and the extract was separated. After the extract was concentrated, it was detected by a high-performance liquid chromatograph equipped with a Chiralcel OD-H chiral column. The conversion of α-bromoacetophenone and the enantiomeric excess of product R-2-bromo-1-phenylethanol were 83.6% and 88.5%, respectively.
Embodiment 2
[0013] Embodiment 2: add 50mL of 0.1mol / L, the phosphate buffer saline solution of pH=6.4 and 10g chopped carrot root cells in an Erlenmeyer flask, vibrate (120r / min ) for 20 min to activate the carrot root cells, then add 0.02 g (0.4 g / L) α-bromo-p-methylacetophenone, and shake and react at the same temperature for 28 h. After the reaction, 40mL of ethyl acetate was added to the reaction solution, centrifuged after shaking and extraction, and the extract was separated. After the extract was concentrated, it was detected by a high-performance liquid chromatograph equipped with a Chiralcel OD-H chiral column. The conversion of α-bromo-p-methylacetophenone and the enantiomeric excess of the product R-2-bromo-1-p-methylphenethyl alcohol were 63.1% and 88.9%, respectively.
Embodiment 3
[0014] Example 3: Add 50 mL of 0.1 mol / L, pH=6.4 phosphate buffer solution and 15 g of carrot root cells in an Erlenmeyer flask, shake (120 r / min) in a water bath constant temperature oscillator for 20 min at 30 ° C, Activate the carrot root cells, then add 0.02g (0.4g / L) α-bromo-p-methoxyacetophenone, and shake the reaction at the same temperature for 30h. After the reaction, 40mL of ethyl acetate was added to the reaction solution, centrifuged after shaking and extraction, and the extract was separated. After the extract was concentrated, it was detected by a high-performance liquid chromatograph equipped with a Chiralcel OD-H chiral column. The conversion of α-bromo-p-methoxyacetophenone and the enantiomeric excess of product R-2-bromo-1-p-methoxyphenethyl alcohol were 61.5% and 87.7%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com