Combination therapy of an afucosylated CD20 antibody with bendamustine
A technology of fucosylation and bendamustine, applied in the direction of antibody medical components, antibodies, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0131] Experimental protocol
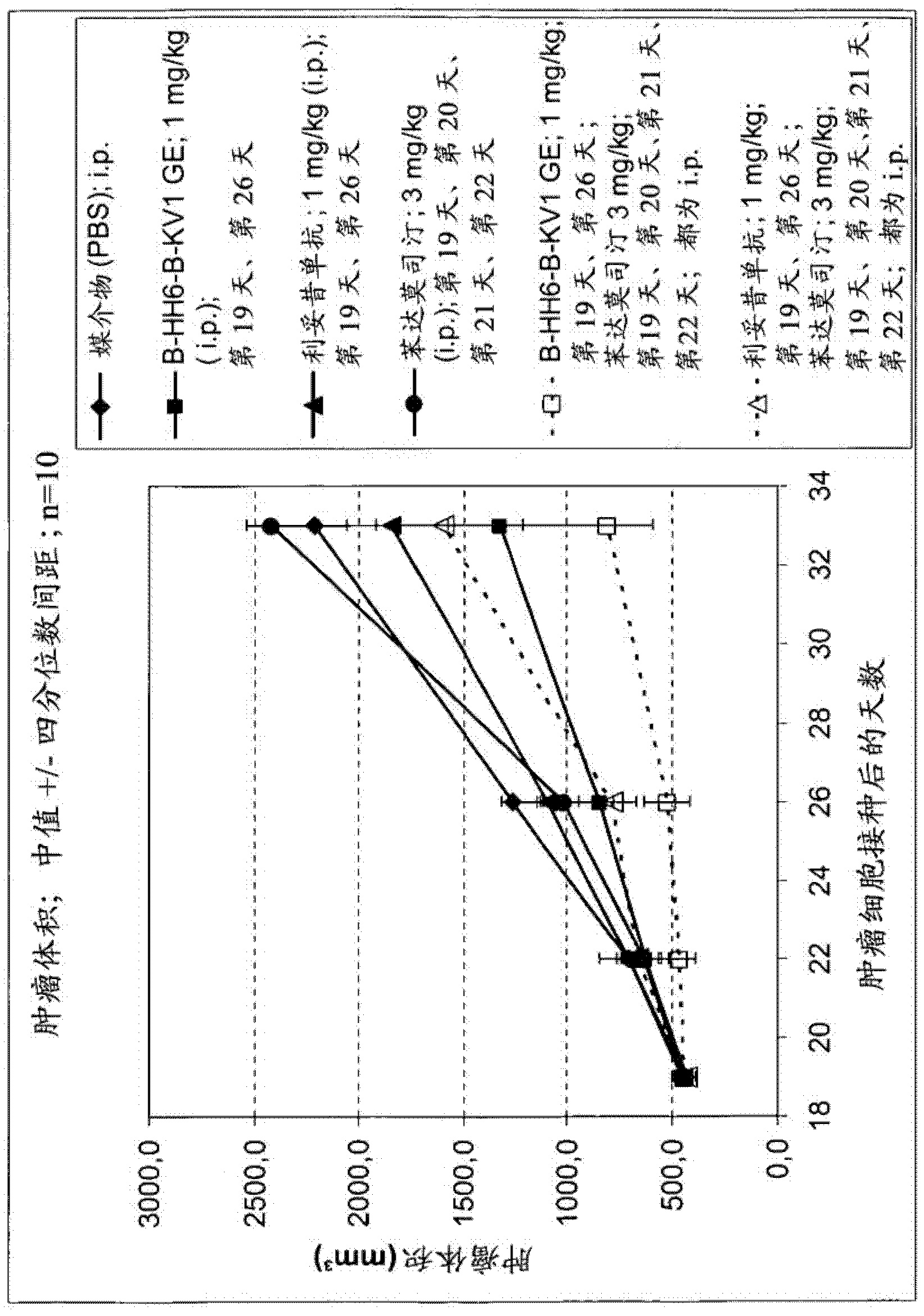
[0132] Antitumor activity of combination therapy with type II anti-CD20 antibody (B-HH6-B-KV1GE) and bendamustine
[0133] test agent
[0134] Afucosylated anti-CD20 antibody B-HH6-B-KV1 GE (afucosylated humanized B-Ly1, glycoengineered B-HH6-B-KV1, see WO 2005 / 044859 and WO 2007 / 031875) are provided as stock solutions (9.4 mg / ml) from GlycArt, Schlieren, Switzerland. Antibody buffer contains histidine, trehalose and polysorbate 20. Antibody solutions were appropriately diluted in PBS from stock for prior injection.
[0135] Clinical grade rituximab (Mabthera) was obtained from Hoffmann La Roche, Basel.
[0136] Bendamustine (Ribomustin ) was purchased from Mundipharma GmbH, Limburg an der Lahn, Germany. The required dilutions were adjusted from the 25 mg / ml stock solution made.
[0137] Cell Lines and Culture Conditions
[0138] The human Z138 mantle cell lymphoma cell line was routinely cultured in DMEM supplemented with 10% feta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com