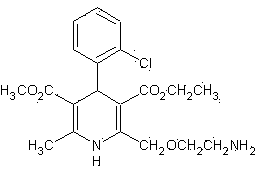
Aspartic acid amlodipine compound and synthesis method thereof
A technology of amlodipine aspartate and a synthesis method, which is applied in the field of amlodipine aspartate compound and its synthesis, can solve the problems of high production risk, low total yield, complicated process and the like, and achieves less toxic and side effects , the route is short, the effect of improving the compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
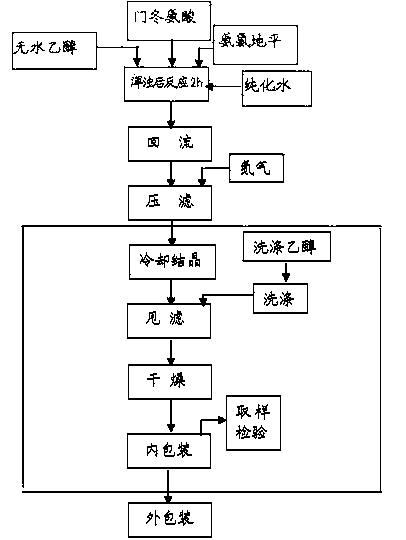
Examples
Embodiment 1
[0036] The specific implementation manner of the present invention will be described in detail below in conjunction with the accompanying drawings.
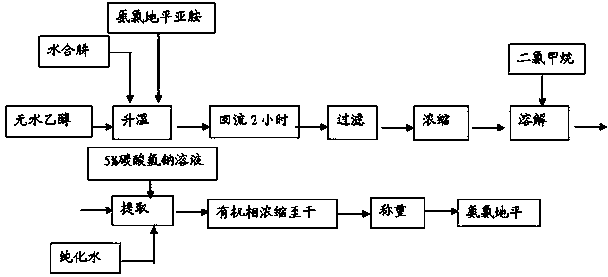
[0037] 1.1 Preparation of amlodipine (such as figure 1 shown):
[0038] 1.1.1 Reaction formula:
[0039]
[0040] 1.1.2. Material ratio:
[0041] Material name Feeding amount moles amlodipine imide 20kg 36.6 Hydrazine hydrate 3.3kg 55.7 Absolute ethanol 150kg
[0042] 1.1.3. Operation process:
[0043] Add 20kg of amlodipine imine, 150kg of absolute ethanol, and 3.3kg of hydrazine hydrate into the reaction kettle, heat up and reflux for 2 hours (TLC detects the end point of the reaction), cool to room temperature, filter, and the filter cake is washed with a small amount of absolute ethanol, and the filtrate is reduced Concentrate to dryness under pressure, add 140kg of dichloromethane to the residue, stir to dissolve, wash with 50kg of water, and then wash once with 30kg of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com