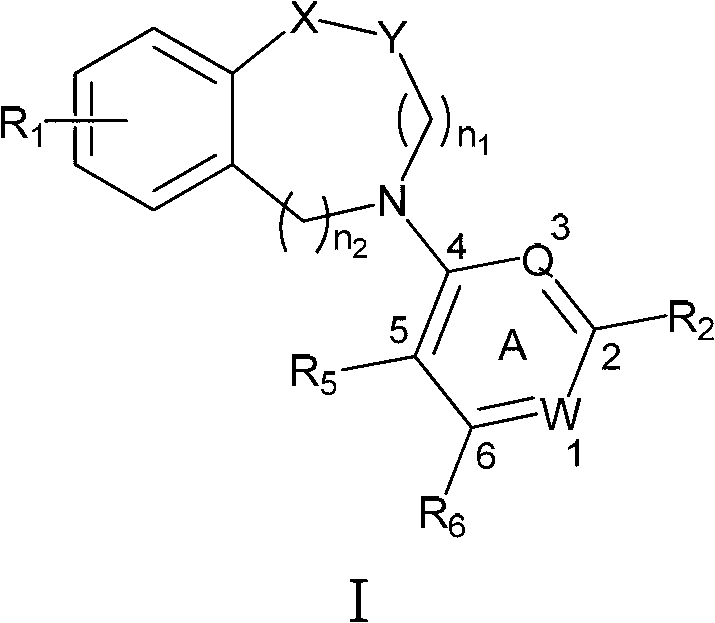
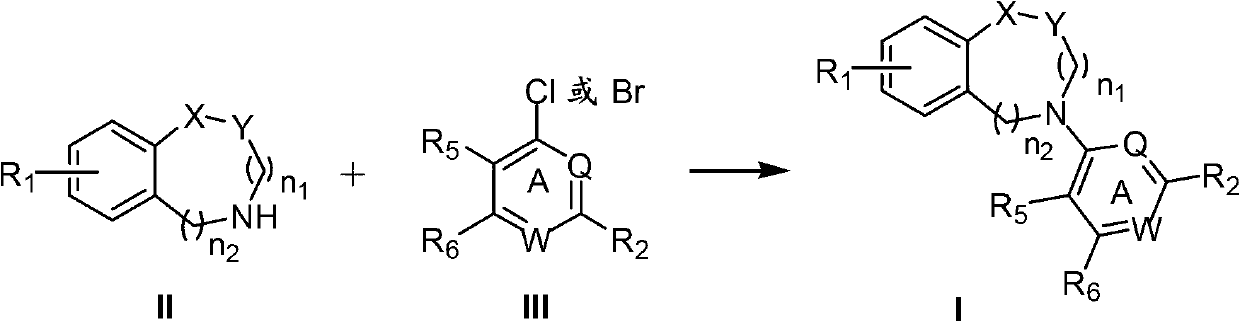
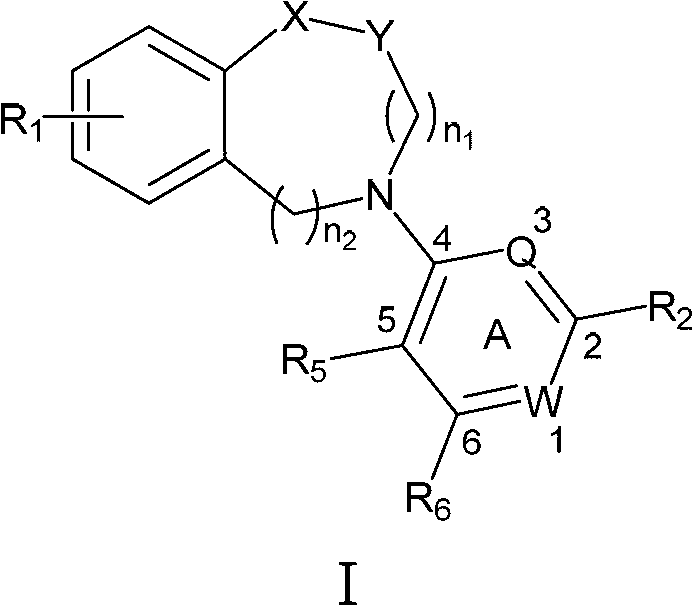
N-aryl unsaturated fused ring tertiary amine compound, preparation method thereof and application thereof to tumor resistance
一种化合物、芳基的技术,应用在N-芳基不饱和稠环叔胺类化合物领域,能够解决生命健康未能达到满意的效果、抗肿瘤和抗癌药物不能满足临床需求等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Example 1: N 1 -[4-(2-chloro)quinazolinyl]-6-methoxy-1,2,3,4-tetrahydroquinoline (compound 1, method one)
[0143]
[0144] The reaction was refluxed for 3 hours. Yellow solid 282mg, yield 87%, melting point 136-138°C; 1 H NMR (CDCl 3 ): δppm 2.12 (2H,m,3'-CH 2 ),2.86(2H,t,J=6.8Hz,4'-CH 2 ),3.81(3H,s,OCH 3 ),4.07(2H,t,J=6.8Hz,2'-CH 2 ),6.55(1H,dd,J=8.8Hz and 2.8Hz,ArH-7'),6.70(1H,d,J=8.8Hz,ArH-8'),6.81(1H,d,J=2.8Hz, ArH-5'),7.13(1H,m,ArH-6),7.32(1H,dd,J=8.8Hz and 1.2Hz,ArH-5),7.63(1H,m,ArH-7),7.78(1H , dd, J=8.4Hz and 1.2Hz, ArH-8). MS m / z(%)326(M+H + ,100),328(M+3 + ,31).
Embodiment 2
[0145] Example 2: N 1 -[4-(2-Methyl)quinazolinyl]-6-methoxy-1,2,3,4-tetrahydroquinoline (compound 2, method 1)
[0146]
[0147] The reaction was refluxed for 3 hours. Yellow solid 242mg, yield 79%, melting point 134-136°C; 1 H NMR (CDCl 3 ): δppm 2.11 (2H,m,3'-CH 2 ),2.73(3H,s,CH 3 ),2.88(2H,t,J=6.8Hz,4'-CH 2 ),3.79(3H,s,OCH 3 ),4.05(2H,t,J=6.8Hz,2′-CH 2 ),6.53(1H,dd,J=9.2Hz and 2.8Hz,ArH-7'),6.63(1H,d,J=8.4Hz,ArH-8'),6.78(1H,d,J=2.8Hz, ArH-5'),7.12(1H,m,ArH-6),7.32(1H,dd,J=8.4Hz and 1.2Hz,ArH-5),7.62(1H,m,ArH-7),7.79(1H , d, J=8.0Hz, ArH-8). MS m / z(%)306(M+H + ,100).
Embodiment 3
[0148] Example 3: N 1 -[4-(2-Methoxy)quinazolinyl]-6-methoxy-1,2,3,4-tetrahydroquinoline (compound 3)
[0149]
[0150] 2-Chloro-4-(6-methoxy-3,4-dihydroquinolin-1(2H)-yl)quinazoline (81mg, 0.25mmol) and NaOCH 3(40mg, 0.75mmol) was added into anhydrous methanol (4.0ml), and refluxed for 2 hours. Insoluble matter was filtered off, and silica gel column chromatography (eluent: ethyl acetate and petroleum ether gradient elution, ethyl acetate 5%-80%) was separated to obtain 71 mg of a light yellow solid with a yield of 88% and a melting point of 161-163°C. 1 H NMR (CDCl 3 ): δppm 2.09 (2H,m,3'-CH 2 ),2.85(2H,t,J=6.8Hz,4'-CH 2 ),3.80(3H,s,OCH 3 ),4.03(2H,t,J=6.8Hz,2'-CH 2 ),4.09(3H,s,OCH 3 ),6.53(1H,dd,J=8.8Hz and 2.8Hz,ArH-7'),6.67(1H,d,J=8.8Hz,ArH-8'),6.79(1H,d,J=2.8Hz, ArH-5'),6.98(1H,m,ArH-6),7.32(1H,dd,J=8.4Hz and 0.8Hz,ArH-5),7.56(1H,m,ArH-7),7.68(1H , d, J=8.4Hz, ArH-8). MS m / z(%)322(M+H + ,100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com