Method for preparing stable amorphous drug preparation
A technology of pharmaceutical preparations and amorphous state, which is applied in the field of preparation of amorphous pharmaceutical preparations, and can solve problems such as complex processes and unsatisfactory stability of amorphous pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
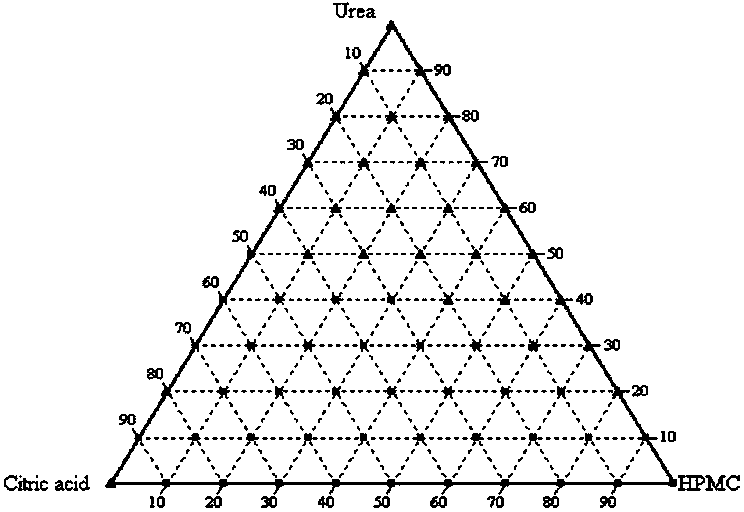
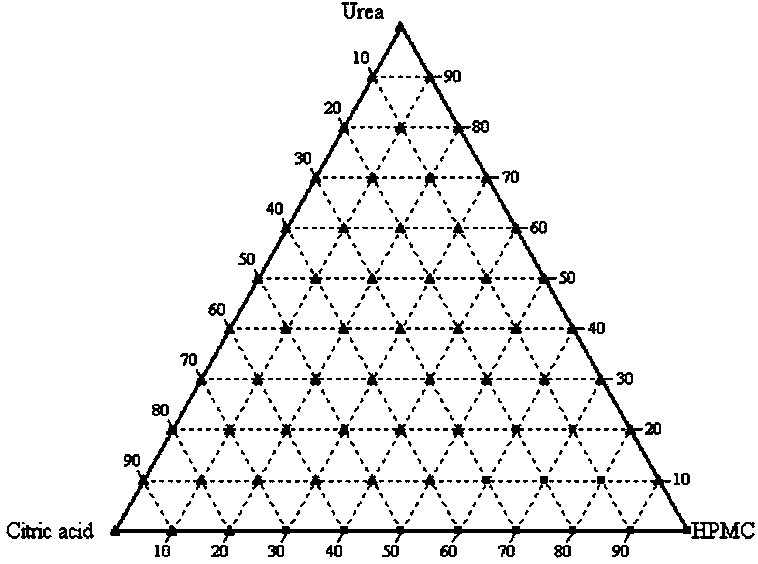
[0026] Example 1: Urea (urea) amorphous preparation
[0027] The molecules of urea are small and crystallize easily. Amorphous urea has not been reported so far. Using the technology of this patent, the amorphous preparation of urea is successfully prepared, and has good stability.
[0028] Prescription Screening:
[0029] 1. Dissolve urea, citric acid (citric acid) and hydroxypropyl methylcellulose (HPMC) in methanol, wherein the amount of citric acid added is 10% to 30% of the total weight, hydroxypropylmethylcellulose The addition amount of element is 60%~80% of total weight;
[0030] 2. The solvent methanol is volatilized at room temperature to obtain an amorphous urea preparation;
[0031] 3. The urea amorphous preparation is stored in a vacuum desiccator; the temperature is 24 ℃ ~ 28 ℃, preferably 25 ℃;
[0032] Microscopy and powder X-ray diffraction were used to check whether a pure amorphous preparation was obtained. figure 1 is the screening phase diagram for...
Embodiment 2
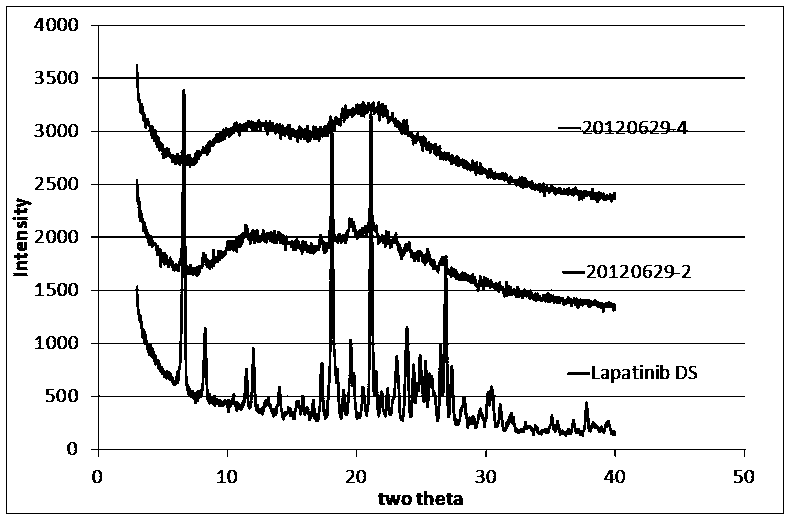
[0034] Embodiment 2: lapatinib amorphous preparation
[0035] Lapatinib is an oral small molecule epidermal growth factor (EGFR: ErbB-1, ErbB-2) tyrosine kinase inhibitor for the treatment of advanced or metastatic breast cancer. Lapatinib is a poorly soluble drug with a solubility of <0.030 mg / mL in water at 25°C. Therefore, the bioavailability of lapatinib is relatively low (<25%). Preparation into an amorphous preparation can increase the solubility of lapatinib, thereby increasing its bioavailability.
[0036] The prescription of lapatinib amorphous preparation is shown in Table 1:
[0037] Table 1 Lapatinib amorphous preparation prescription
[0038] 20120629-2 20120629-4 20120629-6 lapatinib ditoluenesulfonate 100mg 100mg 500mg PVPK30 700mg 600mg 3000mg Citric Acid Monohydrate --- 100mg 500mg
[0039] The preparation method is as follows:
[0040] 1. Dissolve lapatinib, PVPK30 and citric acid monohydrate in a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com