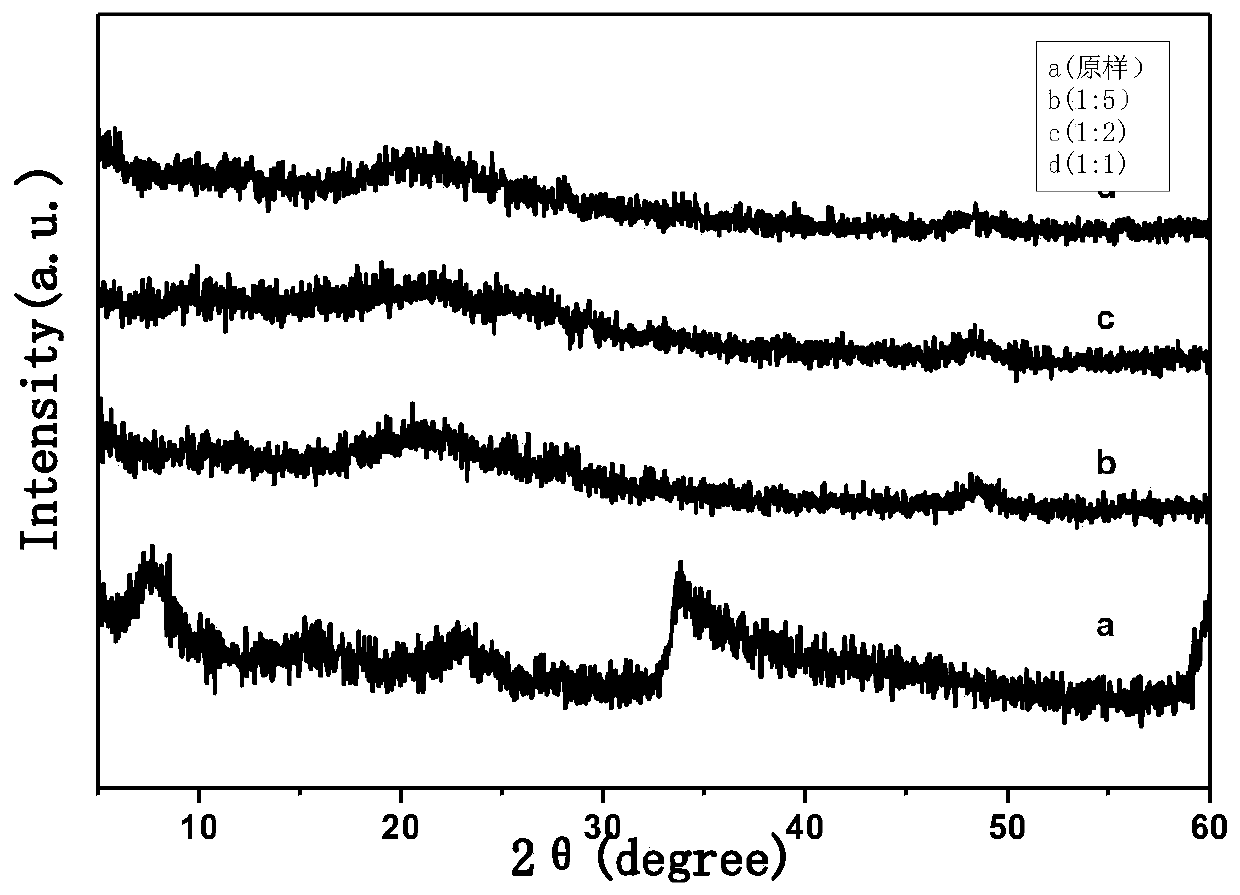
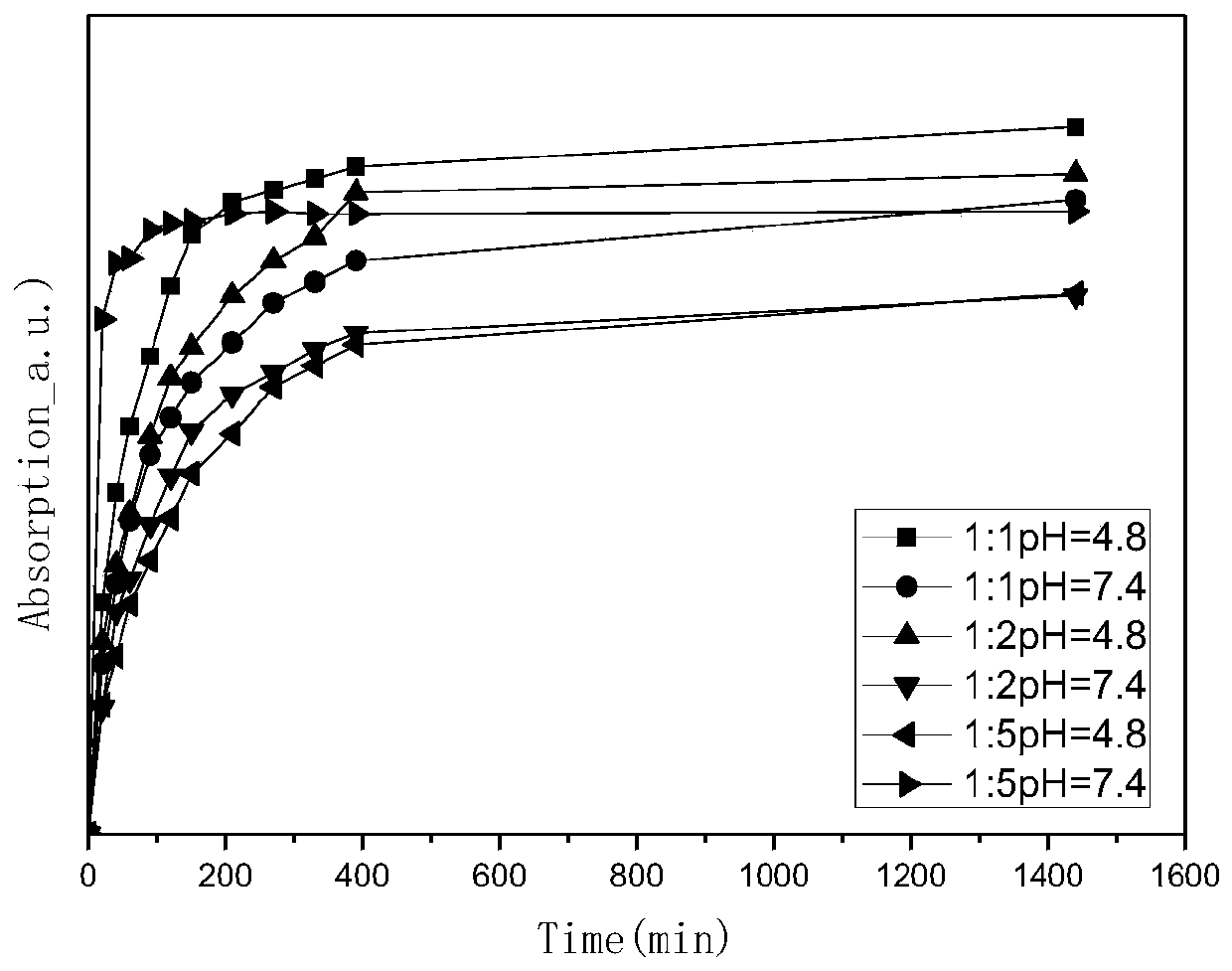
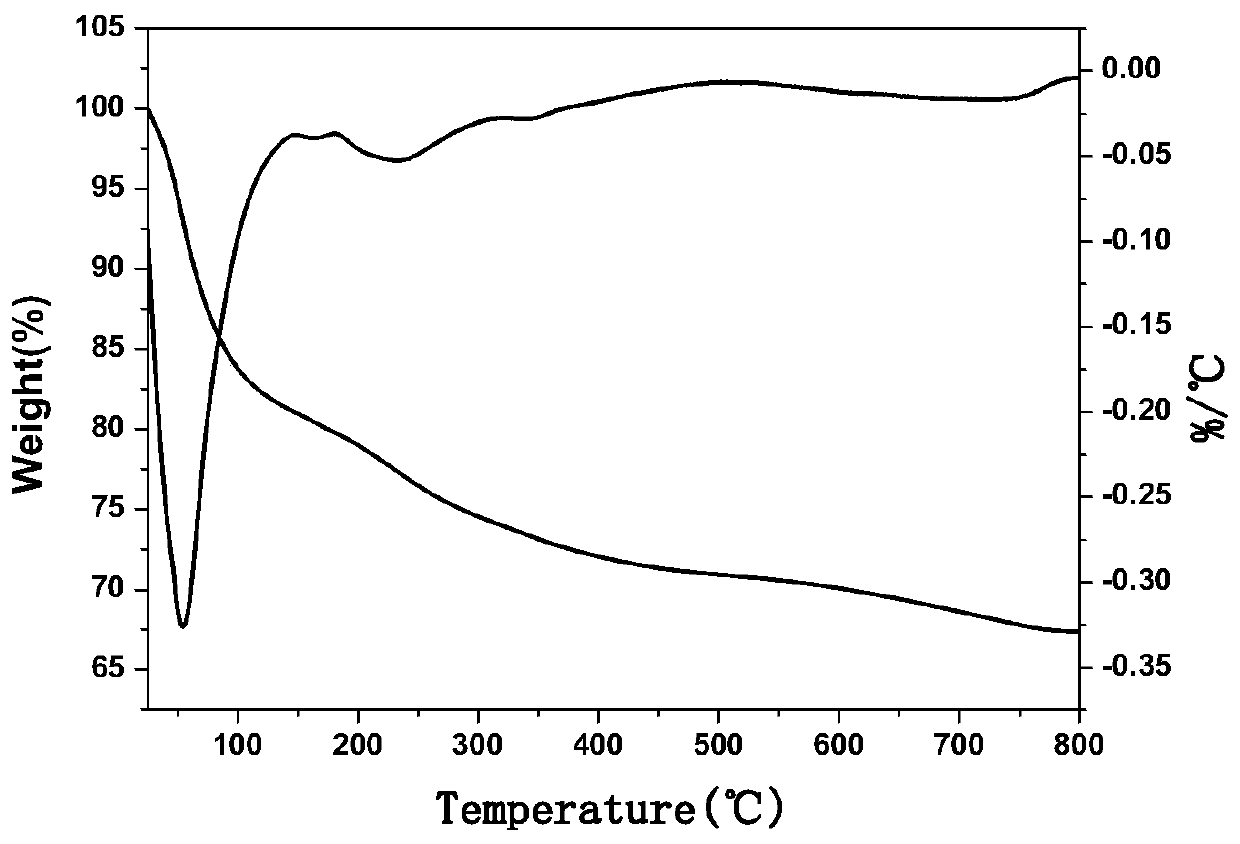
Novel aspirin two-dimensional nano drug-loading and sustained-release system
A technology of aspirin and drug loading, which is applied in the field of medicine to achieve the effects of improving drug burst release, avoiding drug burst release, and prolonging drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Ni-Ti-LDHs
[0031] Nickel nitrate hexahydrate (Ni(NO 3 ) 2 ·6H 2 O), tetrabutyl titanate (Ti(OC 4 h 9 ) 4 ), triethanolamine (N(C 2 h 4 Oh) 3 ), in molar ratio n(Ni 2+ ):n(Ti 4+ ): n(TEA)=5:1:6 was added to the reactor, and in the first step, nitrogen gas was fed into the three-necked flask to drive away the air in the bottle, and 7.5mL of absolute ethanol and 1.390gTi(OC 4 h 9 ) 4 In the flask, stir evenly at 60°C; in the second step, add 3.620g N(C 2 h 4 Oh) 3 , continue to stir evenly; the third step adds 5.930g nickel nitrate (Ni(NO 3 ) 2 ·6H 2O) and 40mL deionized water. Finally use 0.25mol L -1 Sodium hydroxide solution, adjust the pH value of the solution between 8-9. Raise the temperature to 100°C, reflux and condense the reaction system for 45 hours, all the above operations are under N 2 Finished under protection. Suction filter the obtained green mixed solution, wash with a small amount of absolute ethanol to remove the o...
Embodiment 2
[0041] The preparation of Ni-Ti-LDHs and the preparation of Ni-Ti-LDHs nanosheets were all prepared according to the method in Example 1.
[0042] Aspirin-LDHs complex (n 阿司匹林 / n LDHs =1:2) Preparation
[0043] Aspirin was mixed with Ni-Ti-LDHs sample by molar ratio n 阿司匹林 / n LDHs =1:2 for intercalation recombination. First, 20 mL of deionized water was directly added into the Erlenmeyer flask containing 0.3768 g of LDHs, heated and stirred at 60 ° C, 0.050 g of aspirin was added to the above solution and heated and stirred at 70 ° C for 6 h, then stood for 24 h, and then pumped Filter, wash with ice water, and dry under vacuum at 60°C.
[0044] Aspirin-LDHs nanosheet complex (n 阿司匹林 :n LDHs纳米片 =1:2) Preparation
[0045] The Ni-Ti-LDHs nanosheets were peeled off with the aid of formamide ultrasound, and the sol containing LDHs nanosheets was distilled under reduced pressure at 90°C to remove part of the formamide, so that the nanosheets were precipitated, so that the...
Embodiment 3
[0049] The preparation of Ni-Ti-LDHs and the preparation of Ni-Ti-LDHs nanosheets were all prepared according to the method in Example 1.
[0050] Aspirin-LDHs complex (n 阿司匹林 / n LDHs =1:5) preparation
[0051] Aspirin was mixed with Ni-Ti-LDHs sample by molar ratio n 阿司匹林 / n LDHs =1:5 for intercalation compounding. First, 20 mL of deionized water was directly added into the Erlenmeyer flask containing 0.9421 g of LDHs, heated and stirred at 60 ° C, 0.05 g of aspirin was added to the above solution and heated and stirred at 80 ° C for 7 h, then stood still for 25 h, and then pumped After filtration, washing with ice water, vacuum drying at 80°C.
[0052] Aspirin-LDHs nanosheet complex (n 阿司匹林 :n LDHs纳米片 =1:5) preparation
[0053] The Ni-Ti-LDHs nanosheets were peeled off with formamide ultrasonic assistance, and the sol containing LDHs nanosheets was distilled under reduced pressure at 90°C to remove part of the formamide, so that the nanosheets were precipitated to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com