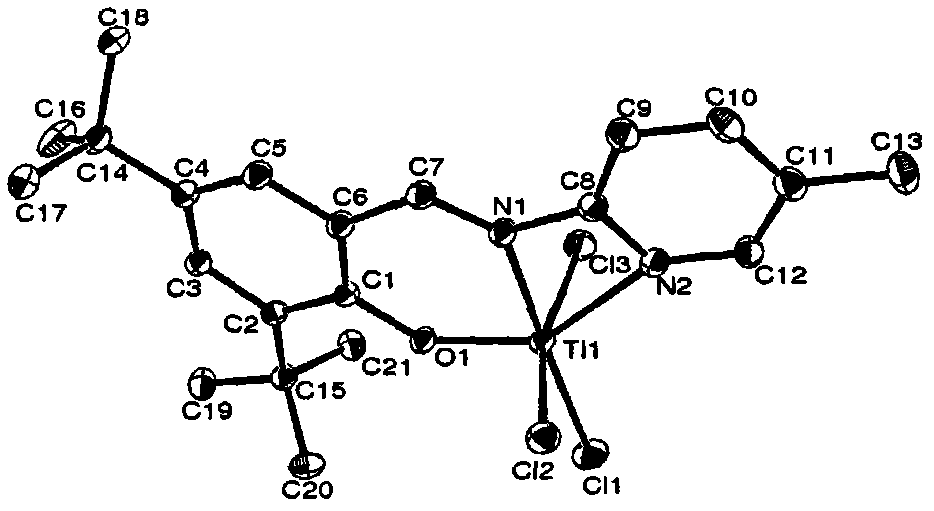
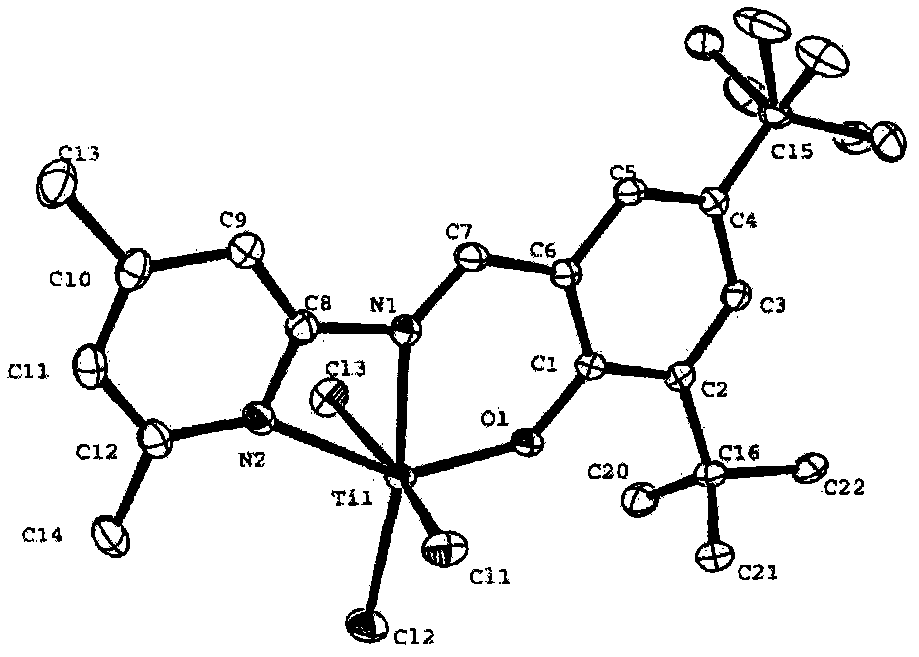
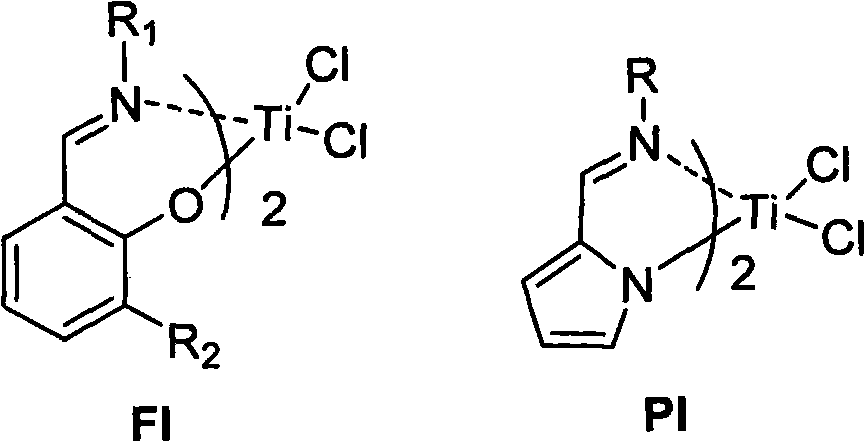
Salicylaldehyde pyridine imine titanium trichloride complex, its preparation method and application, and the method of ethylene polymerization reaction
A technology of salicylaldehyde pyridinimine titanium trichloride and pyridinimine, which is applied in the direction of titanium organic compounds to achieve the effect of high catalytic reaction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The preparation method of the salicylaldehyde pyridine imine titanium trichloride complex provided by the present invention includes the following steps:
[0049] (1) Under the conditions of the aldoamine condensation reaction, the salicylaldehyde of the structure represented by formula (II) and the 2-aminopyridine compound of the structure represented by formula (III) are subjected to the first contact reaction to obtain formula (IV) The pyridine imine compound shown;
[0050]
[0051] Where R 1 -R 8 The definition of is the same as the above formula (I).
[0052] (2) Under the protection of an inert gas, the dry solution of the pyridine imine compound represented by formula (IV) and the metal hydride are subjected to a second contact reaction at a temperature of -25°C to 25°C to obtain a suspension;
[0053] (3) Make the above suspension and TiCl at a temperature of -78℃ to 25℃ 4 ·(THF) 2 After mixing, the third contact reaction is carried out under the conditions of the coor...
Embodiment 1
[0079] 1) Mix 2.34g (10mmol) of 3,5-di-tert-butylsalicylic aldehyde and 0.94g (10mmol) of 2-aminopyridine evenly, and place them in a microwave oven with a frequency of 800MHz. After heating at 200W) for 1 minute, take it out and cool it to room temperature, and then heat it under medium-high fire for 1 minute to obtain red and black liquid. It was recrystallized with ethanol to obtain 2.52 g of orange-yellow crystals of 3,5-di-tert-butylsalicylic aldehyde 2-pyridineimine with a yield of 81.2%.
[0080] Use IR, 1 HNMR, 13 CNMR and elemental analysis were used to characterize the structure of the obtained product. The results are as follows:
[0081] IR(KBr, cm -1 ): 3000(OH)(w), 2960(s), 2906(m), 2867(m), 1610(CH=N)(m), 1579(s), 1461(s), 1432(s), 1360(m), 1198(m), 1169(s), 881(m), 791(m), 769(m), 736(m).
[0082] 1 HNMR: (CDCl 3 , 400MHz, ppm): δ 13.90 (s, 1H, OH), 9.47 (s, 1H, CH=N), 8, 50 (d, 1H, J= 3.60 Hz, Py-H), 7.76 (t, 1H, J = 7.56 Hz, Py-H), 7.49 (s, 1H, Ar-H), 7.35 (d, 1H...
Embodiment 2
[0094] 1) Mix 2.34g (10mmol) of 3,5-di-tert-butylsalicylic aldehyde and 1.08g (10mmol) of 2-amino-5-methylpyridine evenly, place it in a microwave oven with a frequency of 800MHz, and place it under medium-high fire. After heating for 2 minutes, take it out and cool it to room temperature, and then heat it for 2 minutes on a medium-high fire to obtain a red and black liquid. It was recrystallized with ethanol to obtain 2.36 g of orange crystals of 3,5-di-tert-butylsalicylic aldehyde-2-pyridine-5-methylimine with a yield of 73.8%.
[0095] Use IR, 1 HNMR, 13 CNMR and elemental analysis were used to characterize the structure of the obtained product. The results are as follows:
[0096] IR(KBr, cm -1 ): 2997(w), 2958(s), 2906(m), 2868(m), 1612(CH=N)(m), 1570(s), 1462(s), 1386(m), 1358(m ), 1251(m), 1169(s), 1022(m), 881(m), 828(m), 769(w), 680(w).
[0097] 1 HNMR: (CDCl 3 , 400MHz, ppm): δ 13.93 (s, 1H, OH), 9.42 (s, 1H, CH=N), 8.31 (s, 1H, Py-H), 7.56 (d, 1H, J=7.88 Hz, Py-H), 7.46...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com