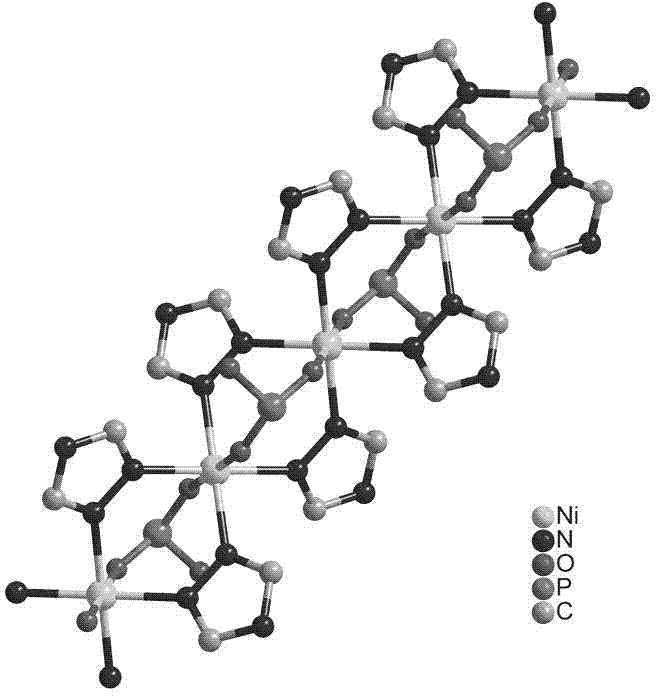
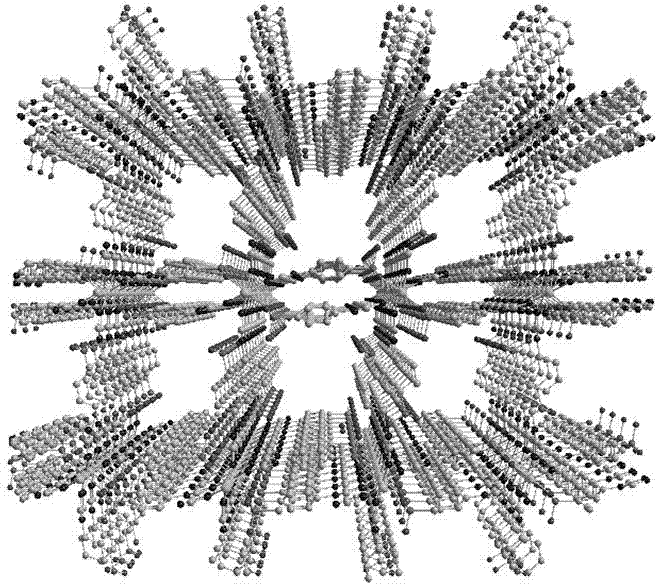
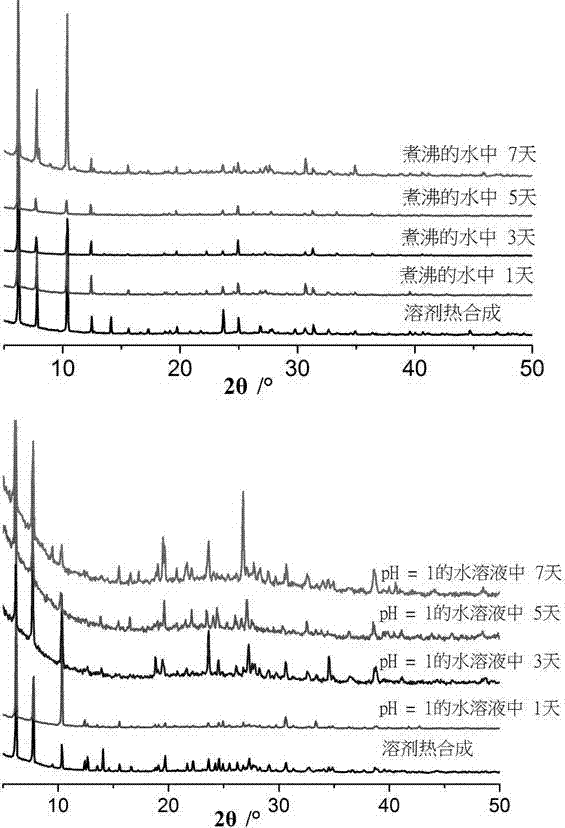
Microporous self-assembly material with stable aqueous phase and based on nitrogen phosphate heterocycle and preparation method for same
A nitrogen-heterocycle, self-assembly technology, applied in the direction of separation methods, chemical instruments and methods, and other chemical processes, can solve the problems of poor application prospects and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 0.131 g of nickel nitrate, 0.454 g of 4-(3,5-dimethyl-1H-1,2,4-triazole-)phenylphosphoric acid to 8 mL of deionized water and 2 mL of N,N'-di In a mixed solvent of methylformamide, after stirring at room temperature for 30 minutes, a green emulsion was obtained, which was transferred to a stainless steel reaction kettle lined with polytetrafluoroethylene, and reacted at 140 ° C for about 72 hours, and then heated for about 5 Cool at a rate of °C / h, filter to obtain green crystals, wash the crystals three times with deionized water, and dry in an oven at 60 °C for 30 minutes to obtain the microporous self-assembled material.
Embodiment 2
[0024] Add 0.114 g of nickel acetate and 0.204 g of 4-(1,2,4-triazole-)phenylphosphoric acid into 10 mL of deionized water, and stir at room temperature for 60 minutes. Move it to a stainless steel reactor lined with polytetrafluoroethylene, react at 180°C for about 48 hours, then cool at a rate of about 5°C / h, filter to obtain green crystals, wash the crystals three times with deionized water, The microporous self-assembled material was obtained by placing it in an oven at 60° C. for 30 minutes and drying it for 30 minutes.
Embodiment 3
[0026] Add 0.263 g nickel sulfate, 0.280 g 4-(3,5-diethyl-1,2,4-triazole-)phenylphosphoric acid to 7 mL of deionized water and 3 mL of N,N'-dimethyl in a mixed solvent of formamide and stirred at room temperature for 30 minutes. Move it to a stainless steel reactor lined with polytetrafluoroethylene, react at 140°C for about 72 hours, then cool at a rate of about 5°C / h, filter to obtain green crystals, wash the crystals three times with deionized water, The microporous self-assembled material was obtained by placing it in an oven at 60° C. for 30 minutes and drying it for 30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com