Novel peptide and use thereof
一种组合物、药学的技术,应用在治疗和/或预防软骨损伤或关节炎的新型肽领域,能够解决不容易获得足够数量的自体细胞等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] [Example 1] Preparation of peptide
[0072] At the request of the present inventors, a peptide having the amino acid sequence shown in SEQ ID NO. 1 (Glu-Leu-His-Leu-Asp) was prepared by Peptron Corporation (South Korea). Specifically, using an automated peptide synthesizer (ASP48S, Peptron Inc.), the amino acid units were linked one by one from the C-terminus by Fmoc SPPS (9-fluorenylmethoxycarbonyl solid-phase peptide synthesis method).
[0073] Use NH 2-His(Trt)-2-chloro-trityl resin, the first amino acid at the C-terminus of the peptide was attached to the resin. All amino acids used to synthesize peptides are protected by trityl (Trt), tert-butoxycarbonyl (Boc), tert-butyl (t-Bu), etc., where the N-terminus is protected by Fmoc, and all amino acids are removed in acid Residues. Using 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) / hydroxybenzotriazole (HOBT) / N-methylmorphine phenoline (NMM) as the coupling reagent. (1) Dissolve th...
Embodiment 2-12
[0074] [Example 2-12] Preparation of Peptides
[0075] The peptides of Examples 2-12 were prepared in the same manner as in Example 1, except that the amino acid sequences listed in Table 1 below were used instead of the amino acid sequences in Example 1.
[0076] Table 1
[0077] Example serial number
Embodiment 13
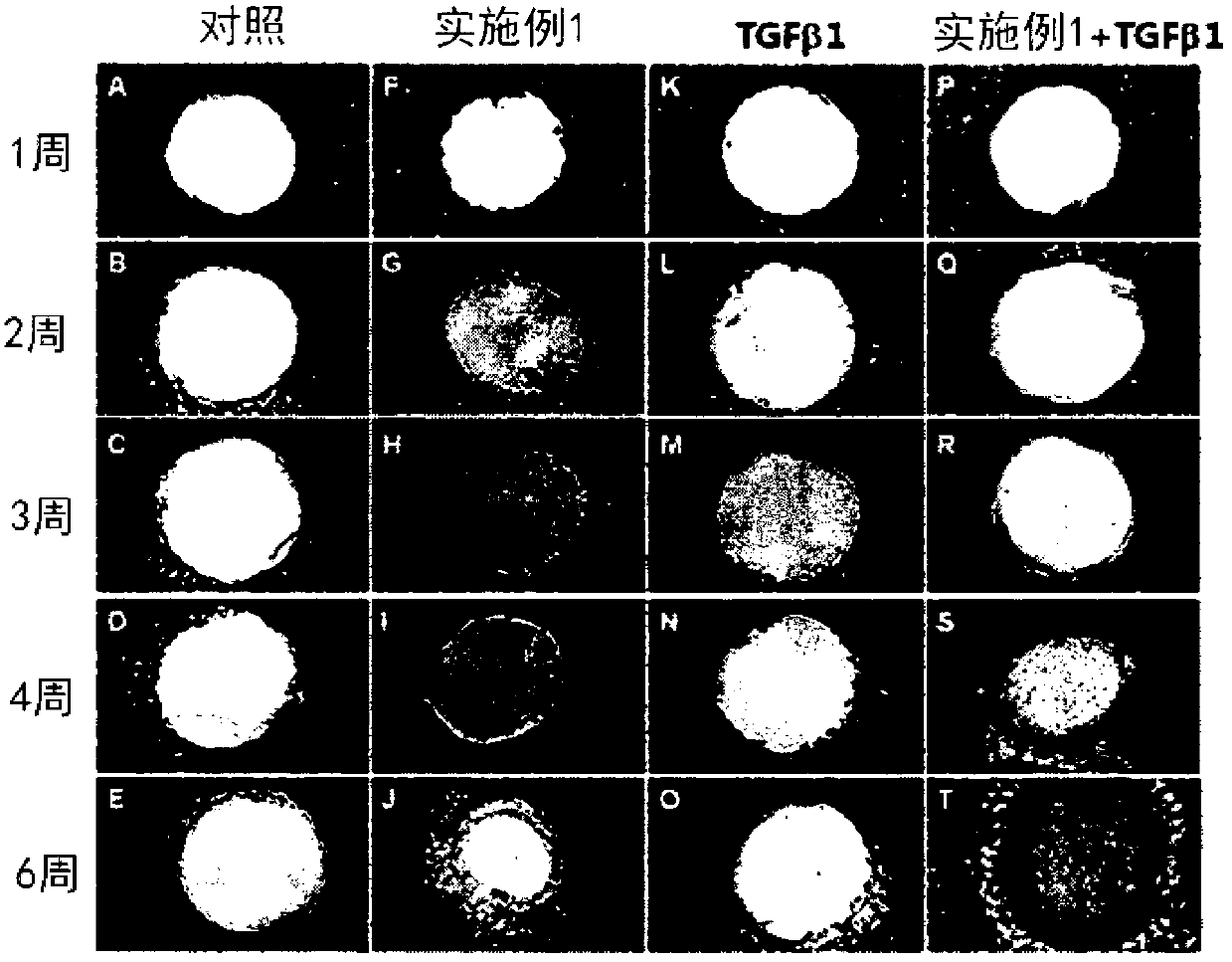
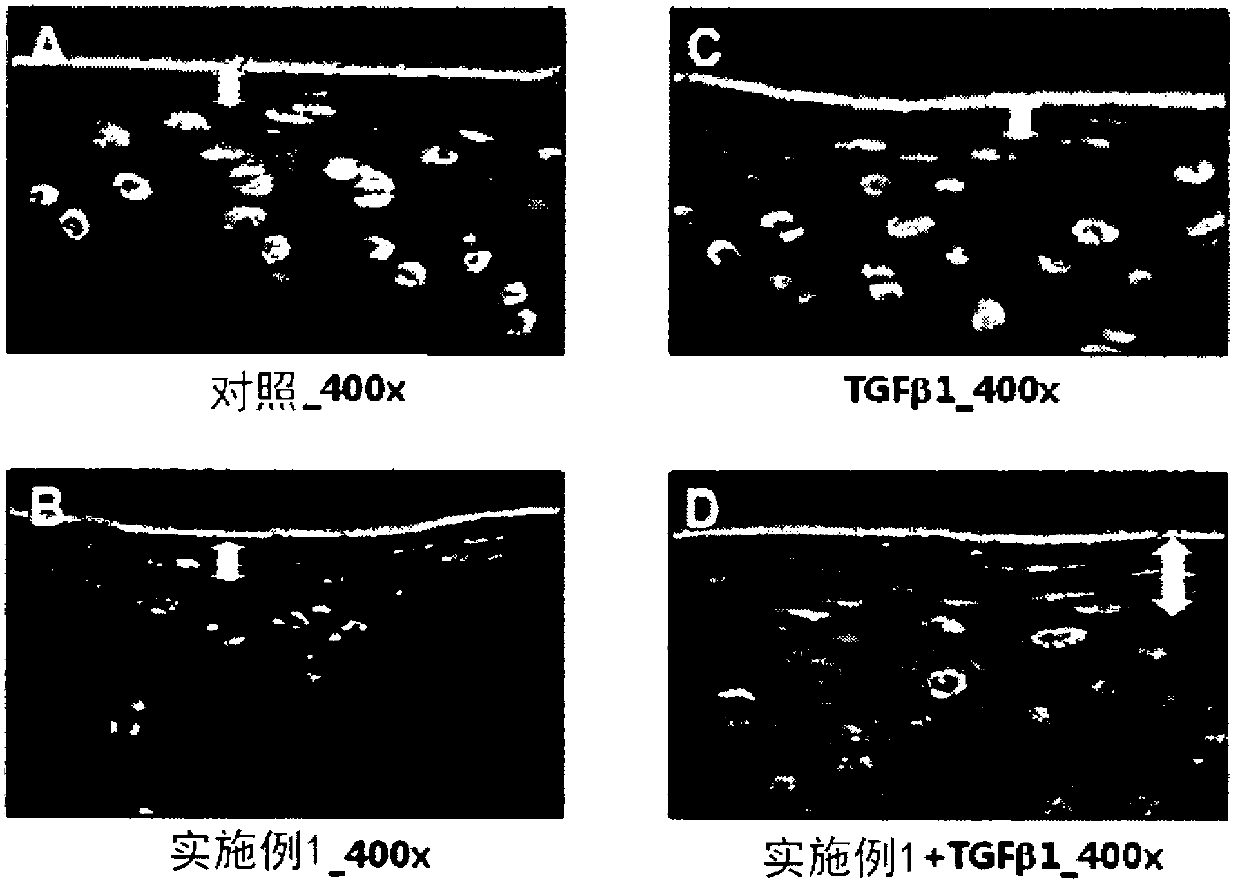
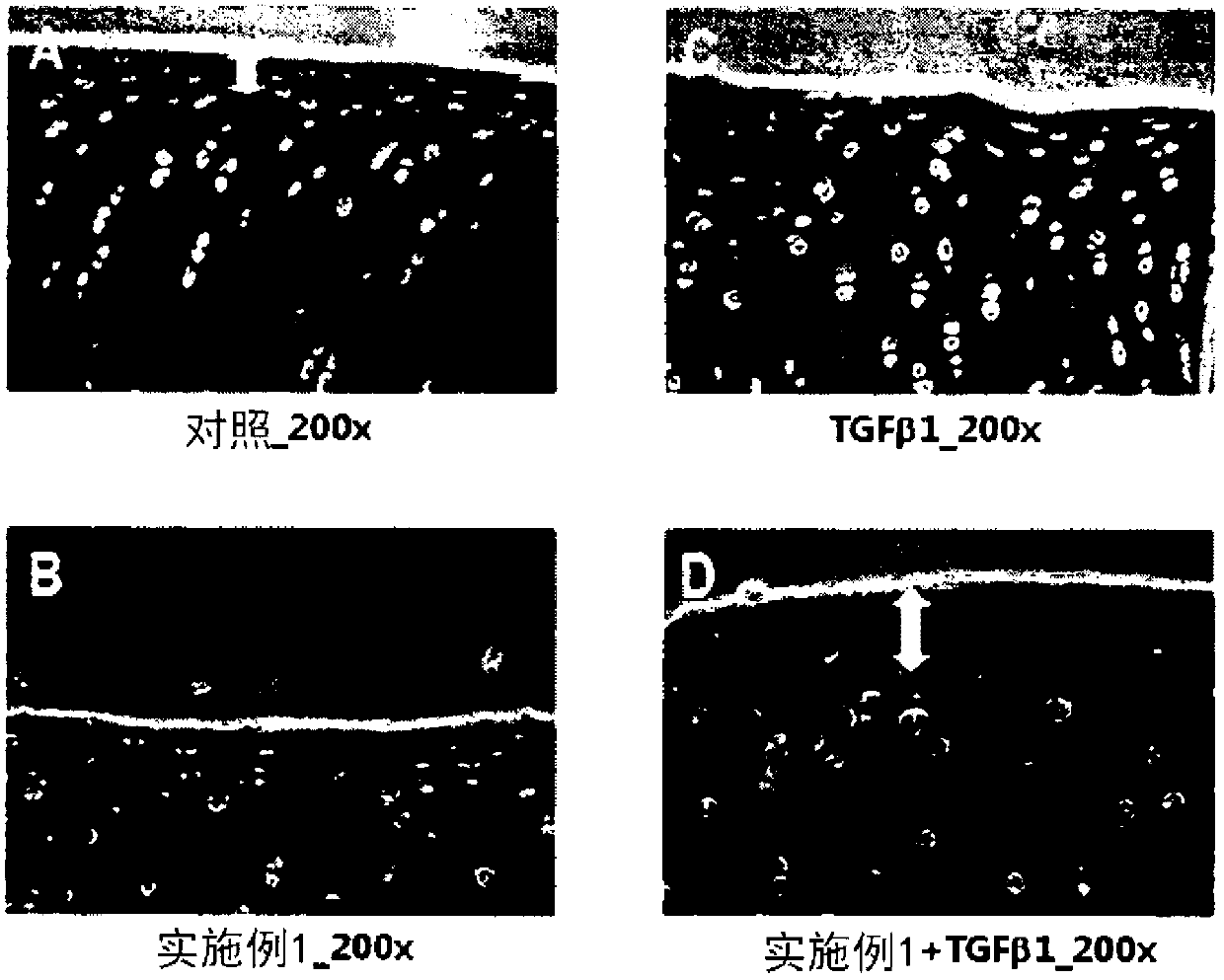
[0078] [Example 13] Confirmation of cartilage regeneration effect with damaged cartilage tissue explants
[0079] Articular cartilage explants were prepared from hoof joints of cows younger than 3 years old within one hour of slaughter at a regional slaughterhouse (located in Ojeong-dong, Daejeon, South Korea). For this purpose, the cartilage part other than the bone was evenly excised from the joint using a scalpel, and cut into a size of about 3 mm x 3 mm to prepare cartilage tissue explants. Using a syringe (21G) with a truncated and ground tip, the lesion area was prepared by uniformly making vertical holes in the surface of the center of the explant.
[0080] The perforated tissue explants were divided into 4 groups including the control group. Each group was placed in Dulbecco's modified Eagle's medium / F12 (DMEM / F12, 1:1, Welgene) supplemented with ascorbate (50 μg / ml, Sigma) and 10% fetal bovine serum (FBS, Invitrogen). ); then treated with 25 μM of the peptide of Exa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com