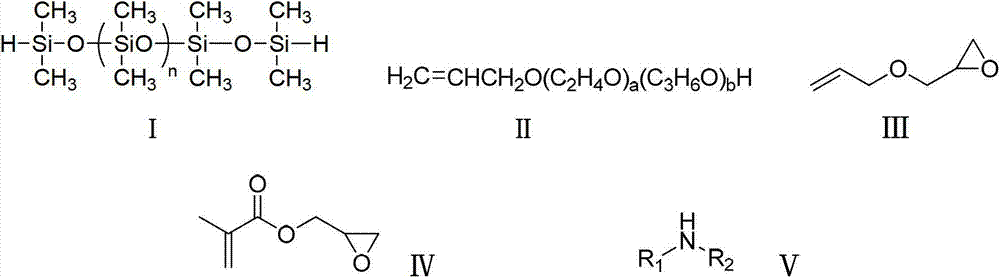Amino modified polysiloxane copolymer and application thereof
A polysiloxane and amino modification technology, which is applied in the direction of organic dyes, can solve the problems of pigment color reduction and application restrictions, and achieve the effect of good flocculation and precipitation and good wetting performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
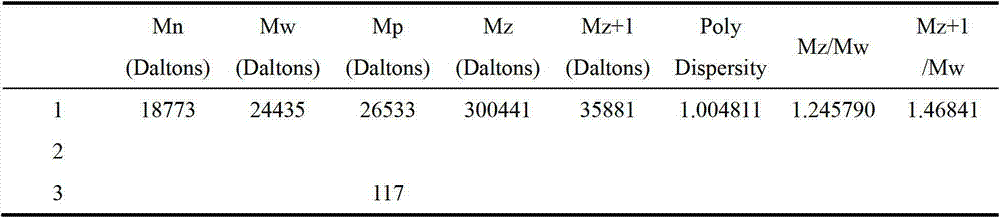
[0033] Add 120g of terminal hydrogen-containing silicone oil (n=8000), 12.5g of allyl polyether (a=200, b=100), and 190g of toluene into a three-necked flask, stir and heat up to 100°C, and add chloroplatinic acid dropwise Isopropanol solution (mass fraction: 1.0%), add 1.4g allyl glycidyl ether after reacting for 3 hours, continue reacting for 5 hours, distill off the solvent and low boilers under reduced pressure, add appropriate amount of methanol to wash, and let stand to remove impurities phase, intermediate A-1 was obtained. The gel chromatography (GPC) test data of intermediate A-1 is shown in Table 1 below.
[0034] IR (tablet method): 3449.59 (s, υ-OH), 2869.86 (s, υCH 2 -O), 1457.30 (w, δ-CH 2 -), 1109.32 (s,υ-CH 2 -O-CH 2 -), 1101.94, 1020.47(s, υ–Si-O-Si-), 1260.95, 803.95(s, δSi-CH 3 ), 855.17 (w, δ-CH 2 OCH 2 -).
[0035] Table 1
[0036]
Embodiment 2
[0038]Put 120g of terminal hydrogen-containing silicone oil (n=12,000), 8.4g of allyl polyether (a=200, b=100), and 160g of toluene into a three-necked flask. Under the protection of nitrogen, stir and heat up to 100°C, add chlorine Isopropanol solution of platinum acid (mass fraction is 1.0%), after 3 hours of reaction, add 2.8g of glycidyl methacrylate to continue the reaction for 5 hours, then distill off the solvent and low boilers under reduced pressure, add an appropriate amount of methanol to wash, and let it stand The impurity phase was removed to obtain intermediate A-2. The GPC test data of Intermediate A-2 is shown in Table 2 below
[0039] IR (tablet method): 3449.59 (s, υ-OH), 2889.06 (s, υCH 2 -O), 1790.03(s, υ-C=O), 1453.70(w, δ-CH 2 -), 1102.39 (s,υ-CH 2 -O-CH 2 -), 1101.91, 1021.21 (s, υ-Si-O-Si-), 1262.42, 803.95 (s, δSi-CH 3 ), 855.73 (w, δ-CH 2 OCH 2 -).
[0040] Table 2
[0041]
Embodiment 3
[0043] Add 120g of terminal hydrogen-containing silicone oil (n=12,000), 8g of allyl polyether (a=150, b=0), and 120g of toluene into a three-necked flask, stir and heat to 100°C, add isopropanol of chloroplatinic acid Solution (mass fraction is 1.0%), add 1.4g allyl glycidyl ether after reacting for 3 hours, continue reacting for 5 hours, distill off the solvent and low boilers under reduced pressure, add an appropriate amount of methanol to wash, stand to remove the impurity phase, and obtain Intermediate A-3. The GPC test data of Intermediate A-3 is shown in Table 3 below
[0044] IR (tablet method): 3450.41 (s, υ-OH), 2867.80 (s, υCH 2 -O), 1458.55 (w, δ-CH 2 -), 1109.72 (s,υ-CH 2 -O-CH 2 -), 1020.29(s, υ–Si-O-Si-), 1261.25, 804.16(s, δSi-CH 3 ), 856.38 (w, δ-CH 2 OCH 2 -)
[0045] The GPC test data is shown in the table below
[0046] table 3
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com