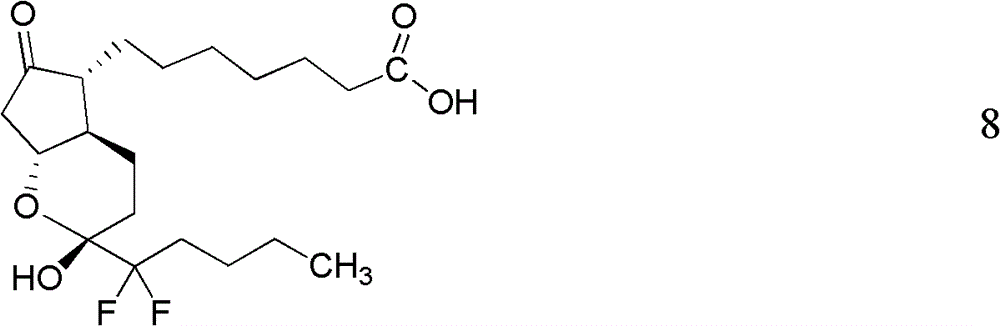
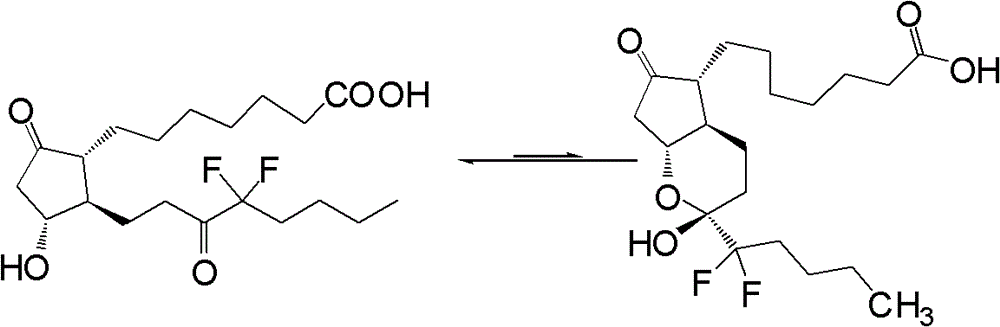
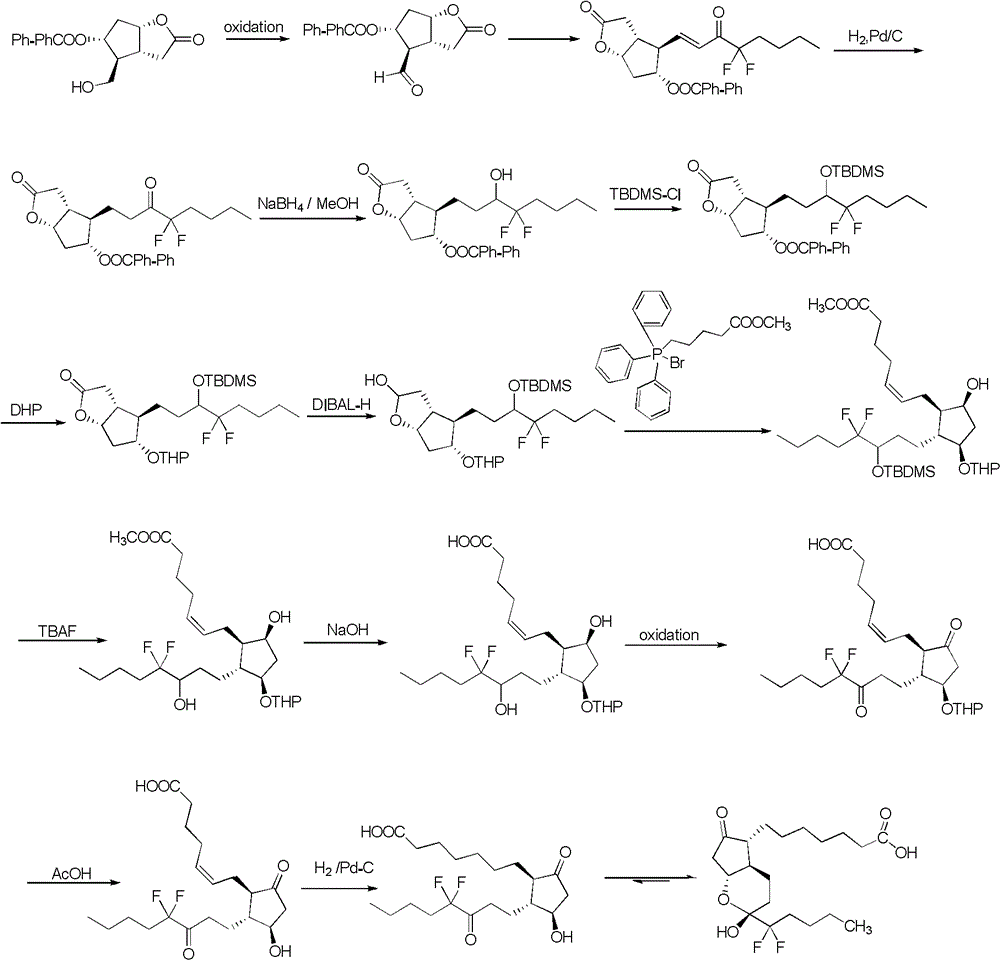
Preparation method of lubiprostone or its intermediate
A technology for lubiprostone and intermediates, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of long steps, expensive upper and side chains, high cost, etc., achieve high reproducibility, increase yield, and save costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]
[0065] Compound 1 (50g, 0.29mol) was dissolved in DMF (200mL), cooled to 0°C, imidazole (47.5g, 0.70mol) was added, and then TBSCl (52.3g, 0.35mol) in DMF (200mL) solution was slowly added to maintain The temperature of the system does not exceed 10°C. After the addition, the reaction is quenched by adding saturated ammonium chloride (200mL) after stirring at 0~5°C for 2h. Extracting with dichloromethane until TLC shows no compound 2 in the water layer, adding citric acid (10 %, 200mL) wash twice, separate the organic phase, wash once with saturated NaCl solution (200mL), wash with saturated sodium bicarbonate solution (200mL), wash with saturated brine (200mL), dry with anhydrous sodium sulfate, spin-evaporate and concentrate the column The product was obtained by chromatography (80 g, yield 96.3%).
Embodiment 2
[0067]
[0068] Compound 2 (82g, 0.286mol) was dissolved in dichloromethane (820mL), stirred to dissolve, put in 2,2,2-trichloroiminoacetic acid-4-methoxybenzyl ester (192g, 80%, 0.544mol) ), and then add CSA (6.64g, 0.0286mol), after the addition, fully react at room temperature for 24h, add saturated sodium bicarbonate solution (200mL), stir well, extract with dichloromethane until TLC shows no compound 3 in the water layer So far, the organic layers were combined, washed with saturated brine (400 mL), dried over anhydrous sodium sulfate, and concentrated by rotary evaporation to obtain product 3 (100 g, yield 86%).
Embodiment 3
[0070]
[0071] Compound 3 (55g, 135mmol) was dissolved in anhydrous toluene (330mL), the temperature was reduced to -70°C under nitrogen protection, DIBAL-H (203mL, 1Mintoluene, 203mmol) was added dropwise, and the dripping was completed within 1.5 hours. Stir at -70°C for 2 hours, TLC indicated that the reaction was complete, methanol was added dropwise to quench the reaction, the cooling bath was removed, saturated potassium sodium tartrate solution (400 mL) was added dropwise, the temperature was raised to room temperature, and the mixture was stirred thoroughly. Extract with dichloromethane until TLC shows no compound 4 in the water layer. Combine the organic layers, wash with saturated brine (400 mL), dry with anhydrous sodium sulfate, and use rotary evaporation to concentrate directly into the next step without purification.
[0072] CEPPA (140g, 317mmol) was suspended in anhydrous THF (200mL), stirred in an ice bath, and LiHMDS (102g, 610mmol) in THF (200mL) solution was s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com