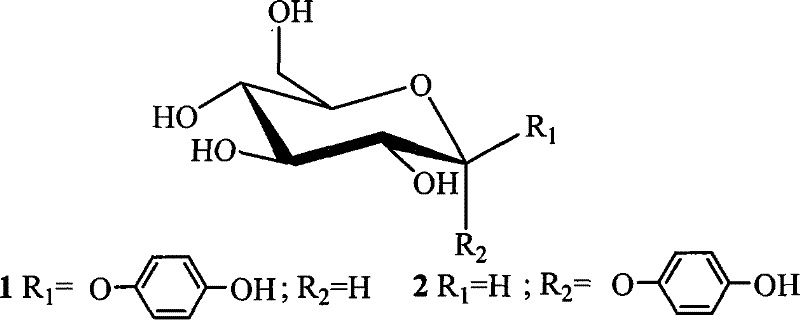
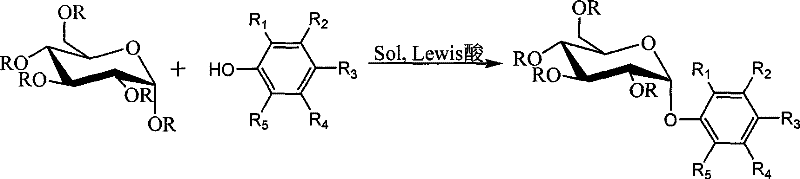
Alpha-arbutin intermediate, 1, 2-cis- indican derivate and stereoselective synthetic method
A technology of stereoselectivity and synthesis method, applied in the direction of sugar derivatives, organic chemistry, etc., can solve the problems of difficulty in the formation of aryl glycosides, a large number of synthesis restrictions, and high reaction conditions, achieve good application prospects, reduce synthesis steps, and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] combine image 3 , the stereoselective synthesis method of α-arbutin intermediate of the present invention, comprises the following steps:
[0038] (1) Add pentabenzoyl glucose donor (chemical structural formula: ) under nitrogen protection,
[0039]
[0040] Hydroquinone acceptor and solvent; wherein, the molar ratio of sugar group donor pentabenzoyl glucose to acceptor hydroquinone is 1:1.5~4, and the amount of solvent is 8~20 of pentabenzoyl glucose times; the solvent is dichloromethane, chloroform or toluene.
[0041] (2) After cooling to -5~5°C, add Lewis acid slowly under stirring and raise to room temperature. Among them, the Lewis acid is BF 3 ·Et 2 O, TMSOTf, AlCl 3 or FeCl 3 .
[0042] (3) Insulate and stir at 40-60°C for 24-72 hours.
[0043](4) the reaction mixture is separated through recrystallization or silica gel column, wherein the eluent separated by silica gel column is ethyl acetate and sherwood oil, and its volume ratio is ethyl acetate: ...
Embodiment 1
[0056] Example 1: Synthesis of p-hydroxyphenyl-2,3,4,6-tetra-O-benzoyl-D-glucoside
[0057] 1.1 Pentabenzoyl Glucose: Hydroquinone: BF 3 ·Et 2 O=1:2:4 (molar ratio), dichloromethane was refluxed for 48h.
[0058] Add 2.5g (3.6mmol) pentabenzoylglucose and 0.79g hydroquinone (7.2mmol) into a 50ml three-necked flask, fill with nitrogen protection, stir and cool to 0°C under ice bath, then add 25ml of dry Chloromethane and 1.9ml (14.4mmol) boron trifluoride ether solution, naturally warmed to room temperature within 2h, then heated to 40-50°C in an oil bath, and refluxed for 48h. After the reaction was completed, add 10ml of dichloromethane to dilute, stir with 20ml of ice water, then wash with 20ml×2 saturated sodium carbonate solution, 20ml of ice water, and dry the organic layer with anhydrous sodium sulfate, filter, evaporate the solvent to obtain a light yellow solid, The yield of crude product is 92%.
[0059] Take 1.3g of the crude product and separate it by silica gel...
Embodiment 2
[0064] Example 2: Synthesis of p-tert-butylphenyl-2,3,4,6-tetra-O-benzoyl-D-glucoside
[0065] Add 2.0g of pentabenzoylglucose and 0.86g of p-tert-butylphenol to a 50ml three-necked flask at room temperature, stir and cool to -5°C under ice bath, then add 20ml of dry dichloromethane and 1.52ml of trifluoro Boronium ether solution, naturally rose to room temperature within 2h, then heated to 40-50°C in an oil bath, and refluxed for 48h. After the reaction is completed, add 10ml of dichloromethane to dilute, stir with 20ml of ice water, then wash with 20ml×2 saturated sodium carbonate solution, 20ml of ice water, dry the organic layer with anhydrous sodium sulfate, filter, evaporate the solvent to obtain a light yellow syrup 2.17 g. Take 0.25g of the syrup and separate it through silica gel column chromatography (ethyl acetate:petroleum ether=1:4~1:2) to obtain 0.22g of glycosidation product with a glycosidation yield of 93%. 1 H-NMR confirmed the C-1 proton integration ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com