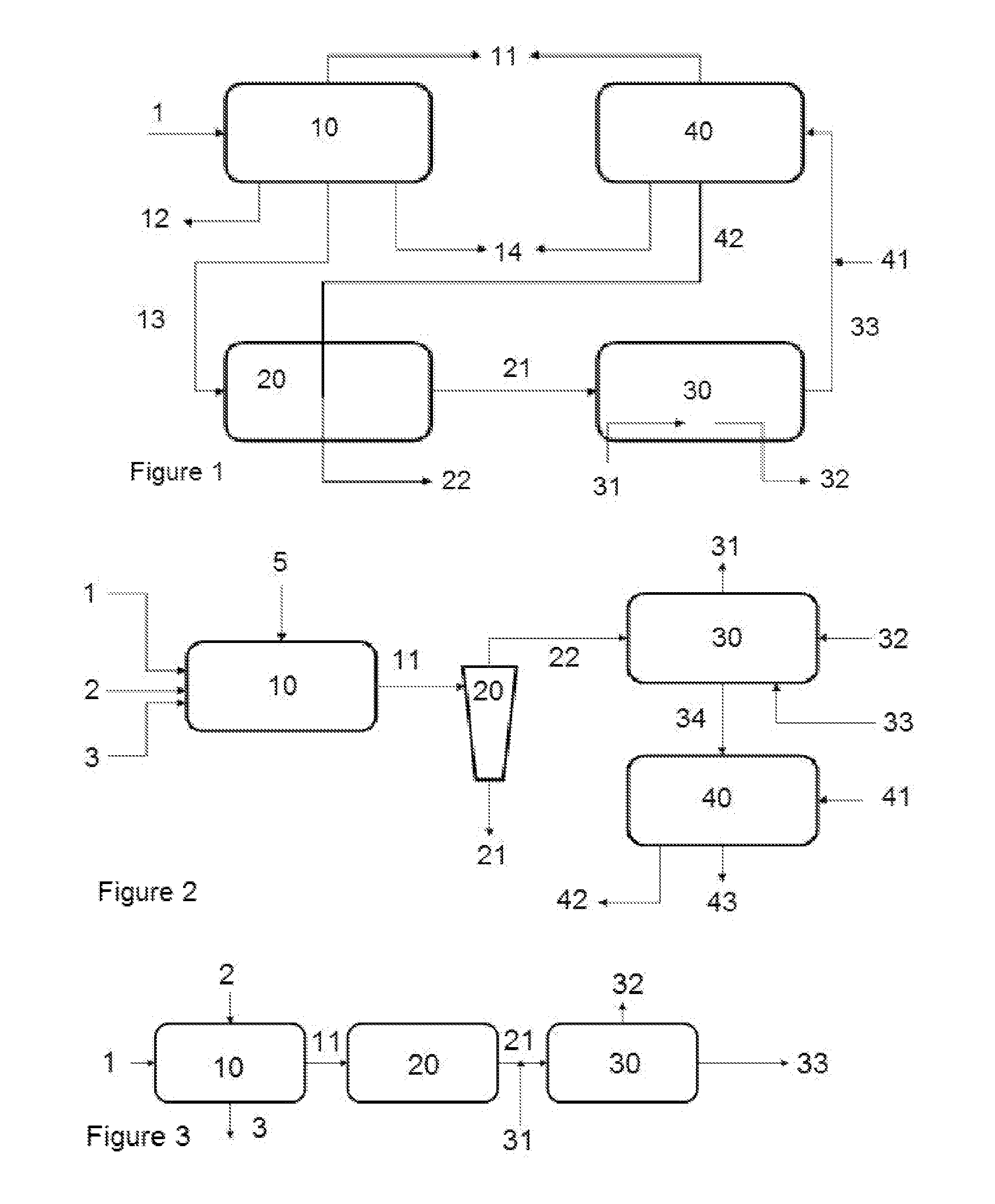
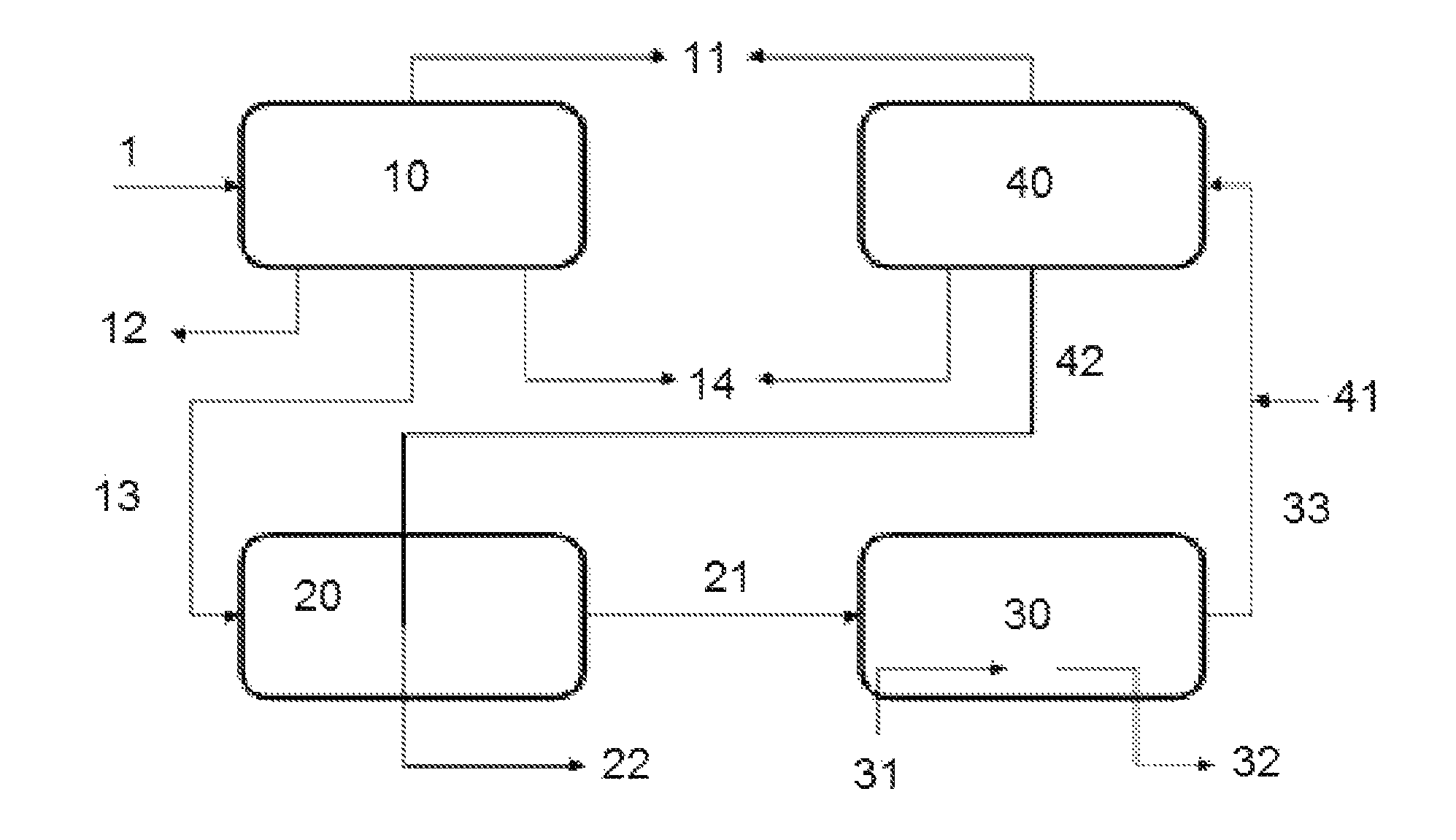
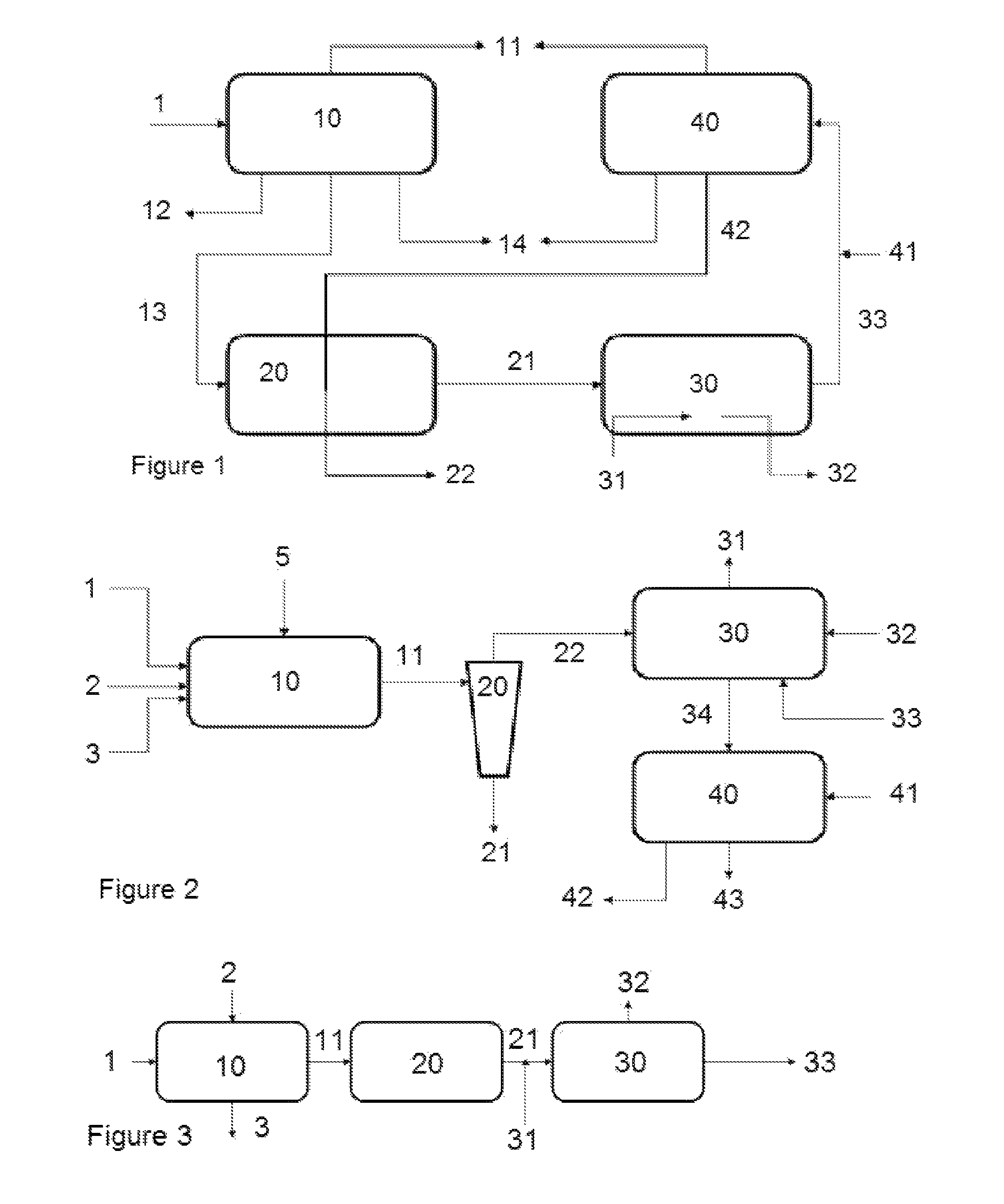
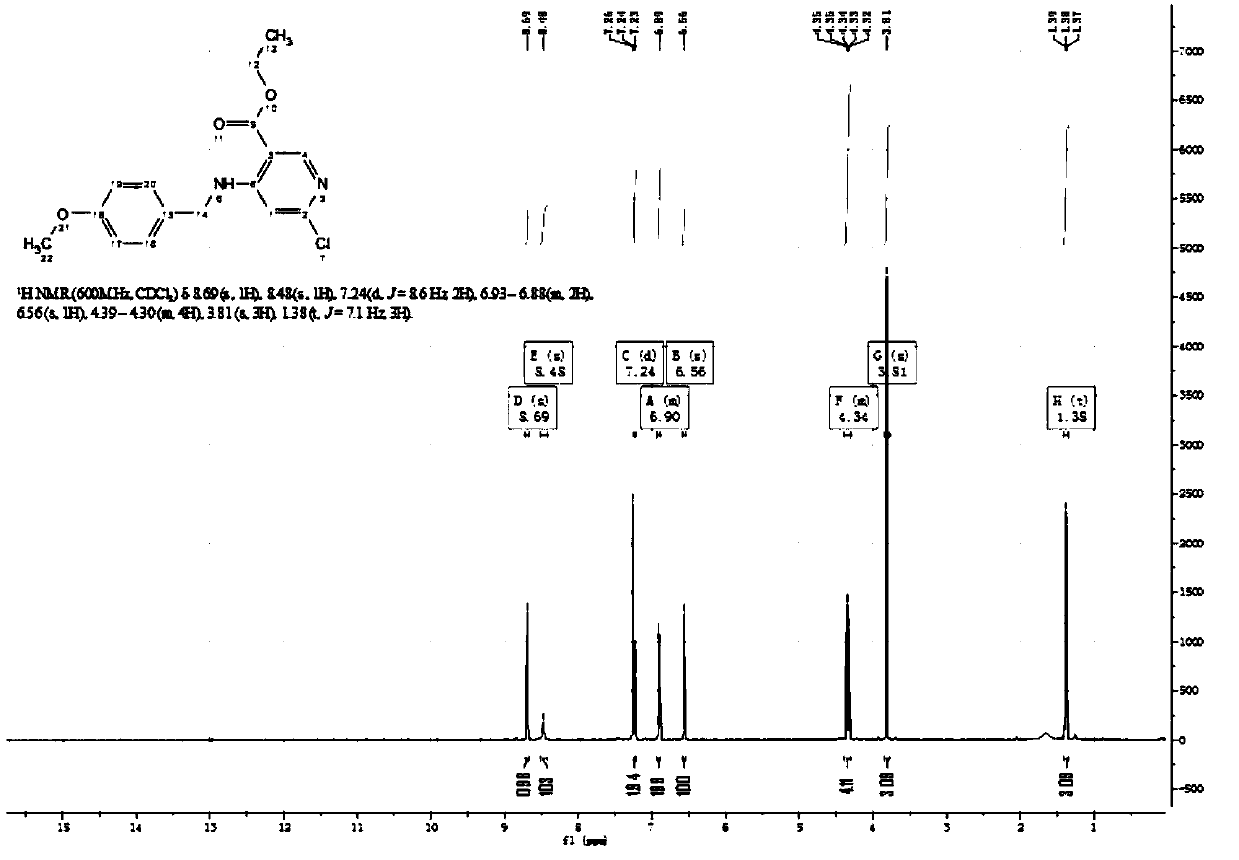
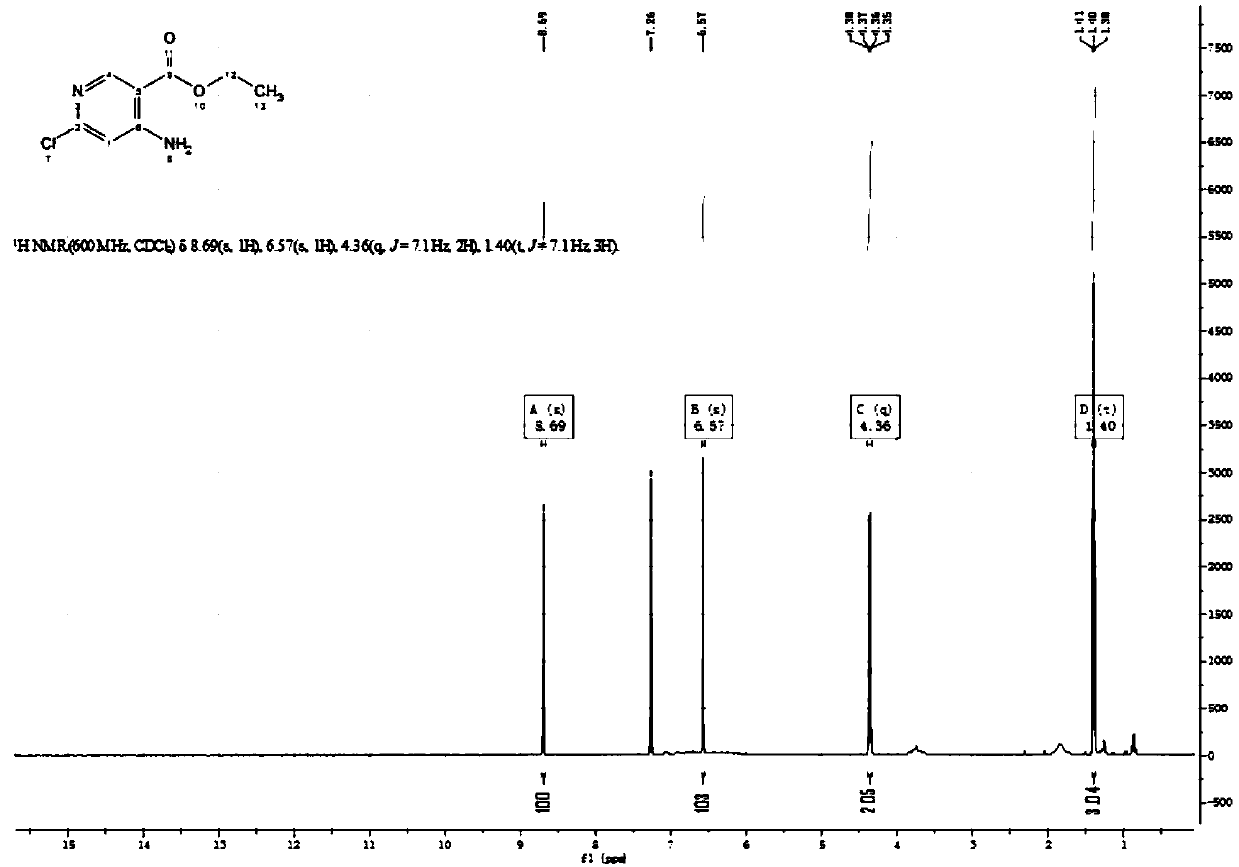
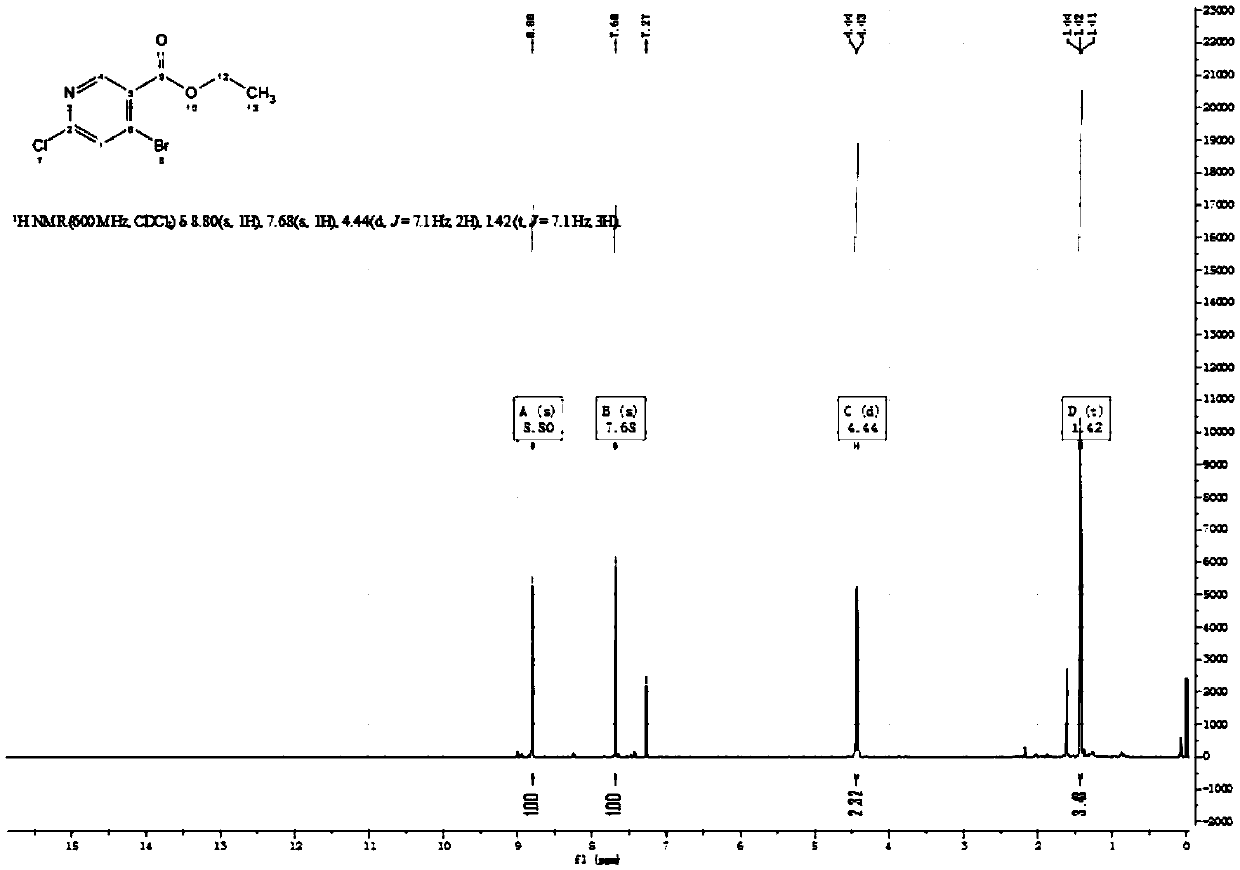
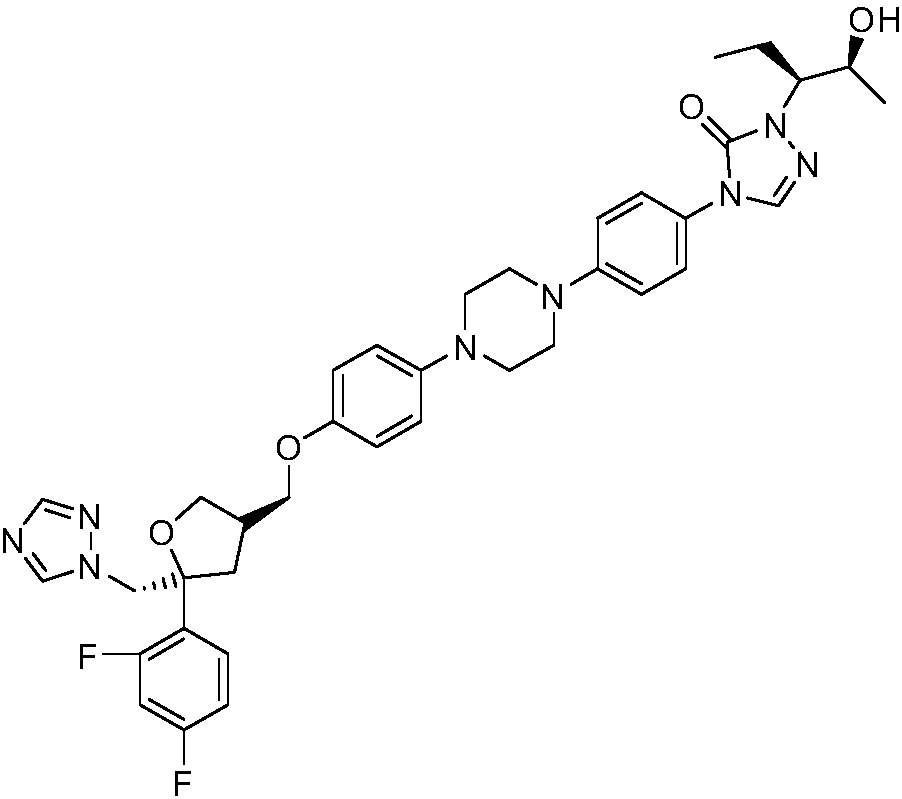
Patents
Literature
34 results about "Diisobutylaluminium hydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH, /ˈdaɪbæl/ DY-bal) is a reducing agent with the formula (i-Bu₂AlH)₂, where i-Bu represents isobutyl (-CH₂CH(CH₃)₂). This organoaluminium compound was investigated originally as a co-catalyst for the polymerization of alkenes.
Process, method, and system for removing heavy metals from fluids
InactiveUS20130306521A1Enhanced surface contactIncrease surface areaRefining with non-metalsRefining with metal saltsThioureaHydrazine compound
Trace amount levels of heavy metals such as mercury in crude oil are reduced by contacting the crude oil with a sufficient amount of a reducing agent to convert at least a portion of the non-volatile mercury into a volatile form of mercury, which can be subsequently removed by any of stripping, scrubbing, adsorption, and combinations thereof. In one embodiment, at least 50% of the mercury is removed. In another embodiment, the removal rate is at least 99%. In one embodiment, the reducing agent is selected from sulfur compounds containing at least one sulfur atom having an oxidation state less than +6; ferrous compounds; stannous compounds; oxalates; cuprous compounds; organic acids which decompose to form CO2 and / or H2 upon heating; hydroxylamine compounds; hydrazine compounds; sodium borohydride; diisobutylaluminium hydride; thiourea; transition metal halides; and mixtures thereof.
Owner:CHEVROU USA INC
Process, method, and system for removing heavy metals from fluids
Owner:CHEVROU USA INC
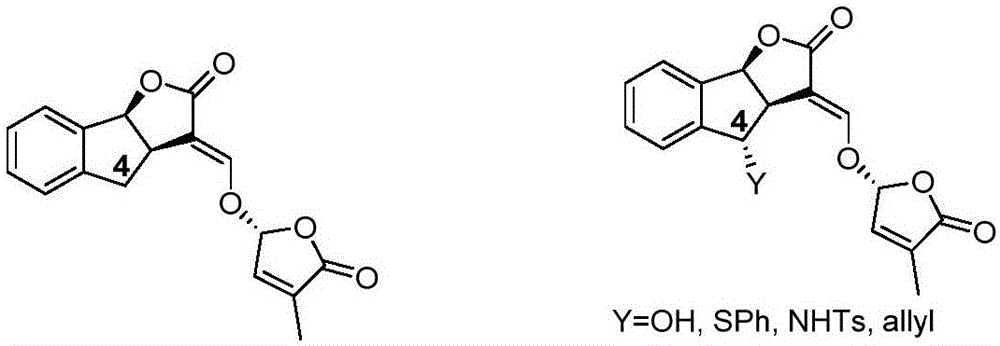
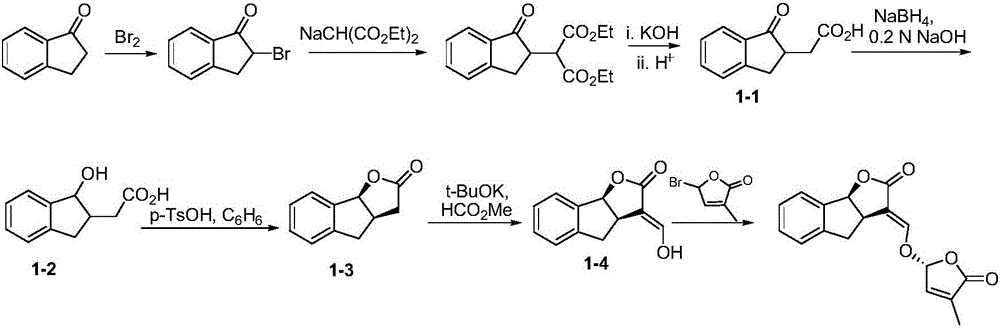
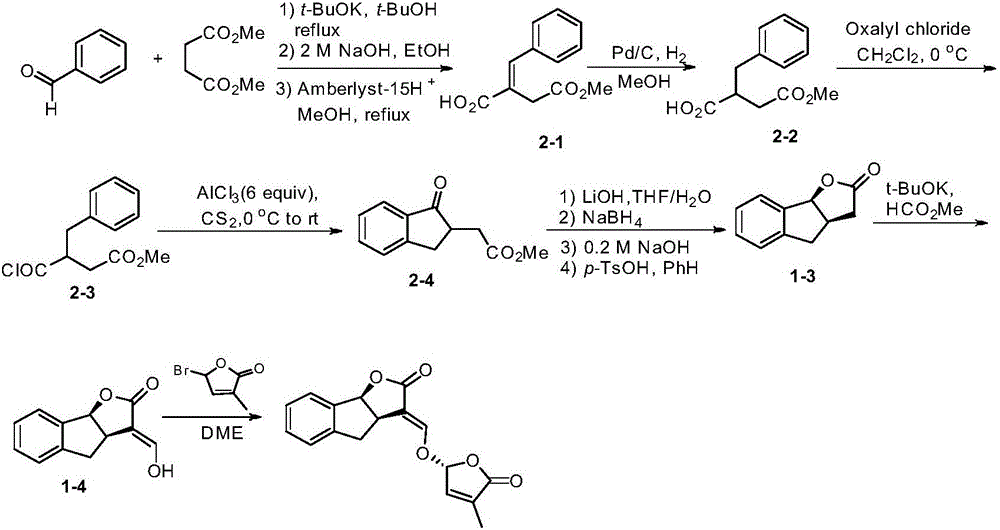
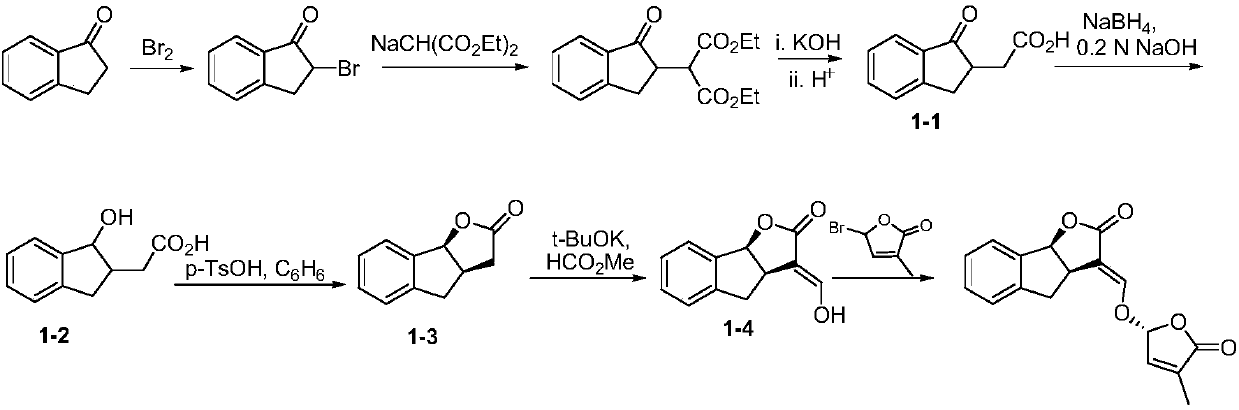
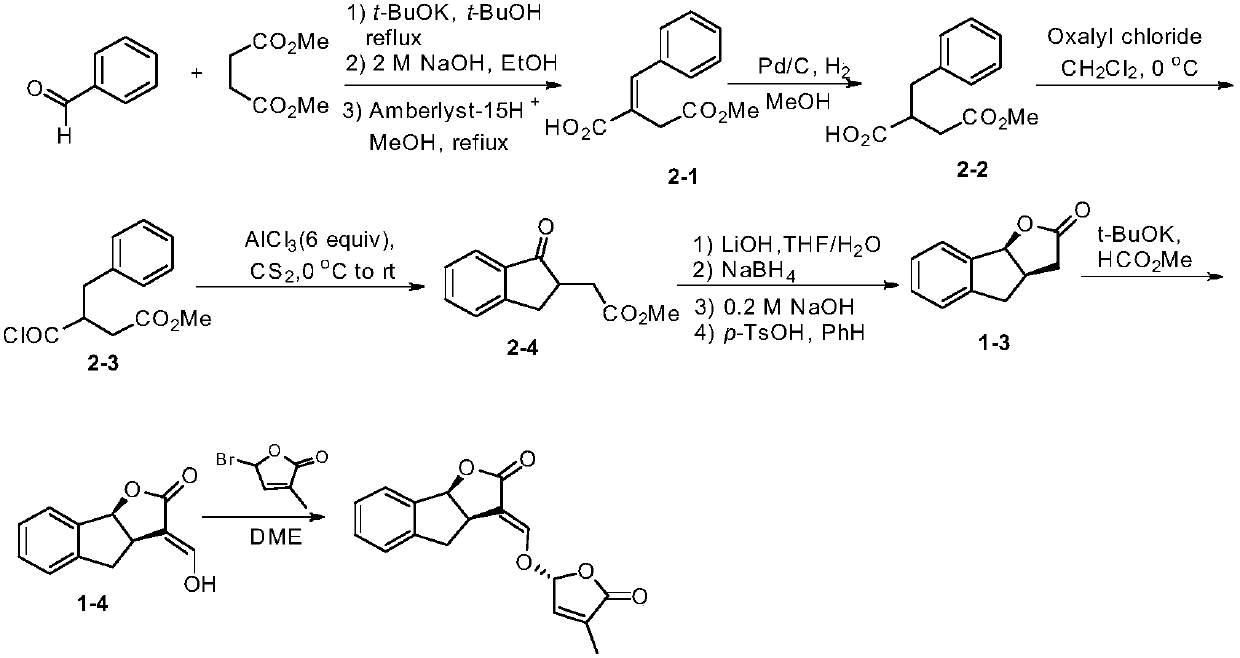
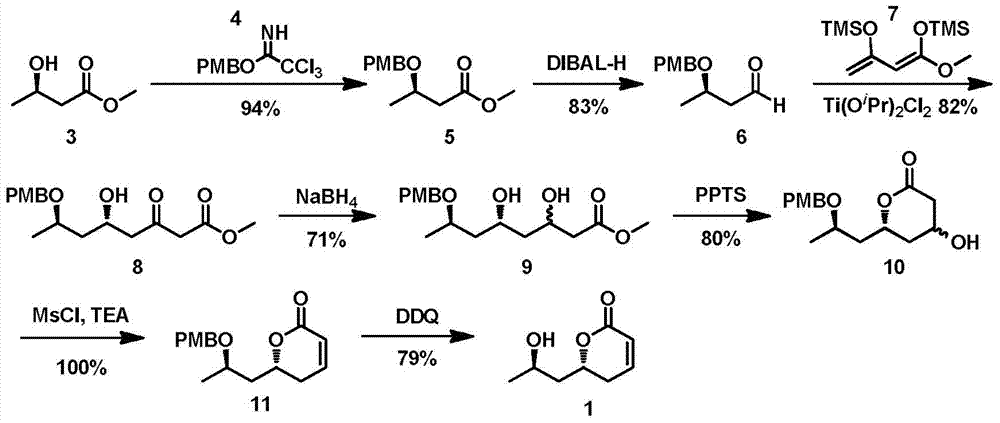
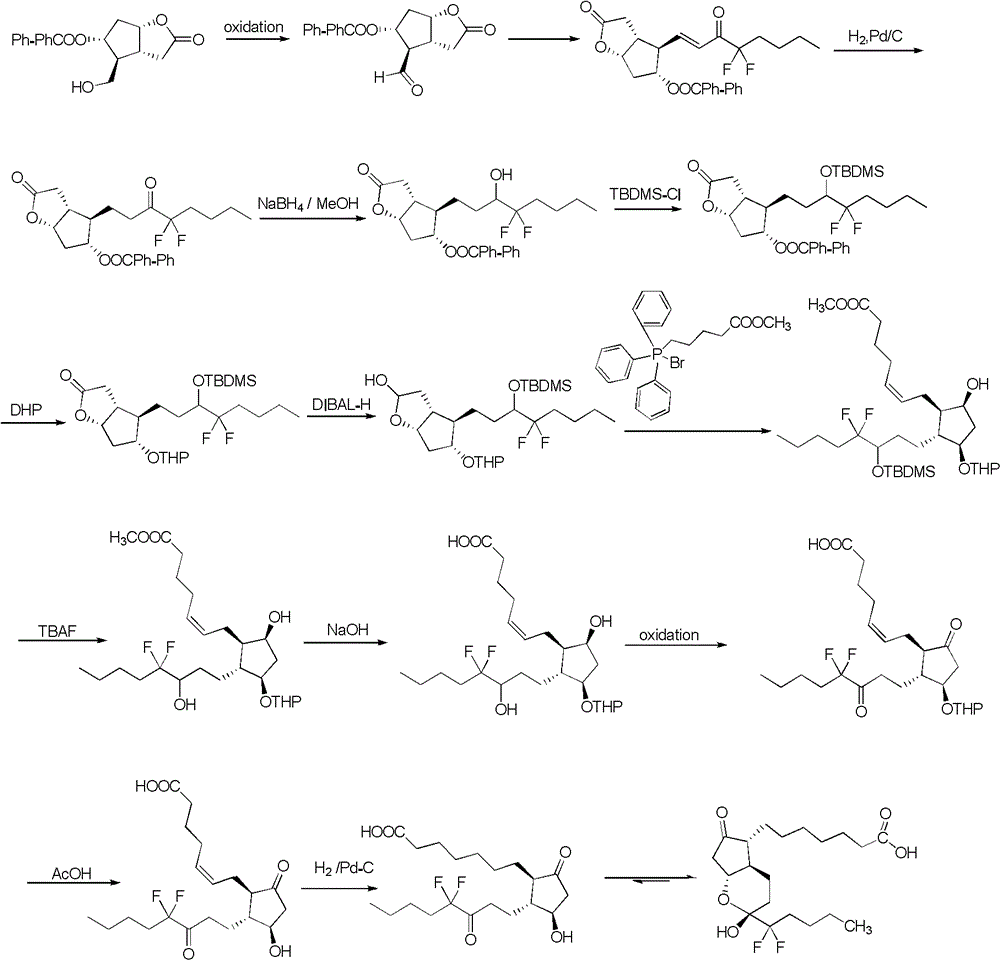
Synthetic method of strigolactone (+/-)-GR24 and 4-substituted (+/-)-GR24
ActiveCN106518822ASimple and fast operationImprove securityOrganic chemistryBenzoic acidFormylation reaction
The invention discloses a synthetic method of strigolactone (+ / -)-GR24 and 4-substituted (+ / -)-GR24. The method comprises the following steps: benzoic acid which is used as a raw material reacts with dibromomethane to obtain an intermediate as shown in the formula I; the intermediate I undergoes diisobutylaluminium hydride reduction to obtain an intermediate as shown in the formula II; the intermediate II and (2-carboxyethyl)triphenylphosphonium bromide undergo coupling under the action of alkali so as to obtain an intermediate as shown in the formula III; the intermediate III reacts with Dess-Martin periodinane to obtain an intermediate as shown in the formula IV; the intermediate IV undergoes cyclization under the action of an acid catalyst or reacts with a nucleophilic reagent so as to obtain ABC tricyclic lactone, namely an intermediate as shown in the formula V; the intermediate V and ethyl formate undergo a formylation reaction to obtain an intermediate VI; and the intermediate VI and bromobutenolide undergo coupling so as to obtain (+ / -)-GR24 and 4-substituted (+ / -)-GR24. The raw materials used in the synthetic method are cheap and easily available; the operation is simple; reaction conditions are mild; safety is high; the catalyst used in the invention is environment-friendly; tricyclic lactone can be constructed by one-step tandem cyclizaiton reaction; the reaction route is short; and overall yield is high. Thus, total cost is greatly reduced. The synthetic method meets requirements of mass synthesis.
Owner:SHAANXI NORMAL UNIV
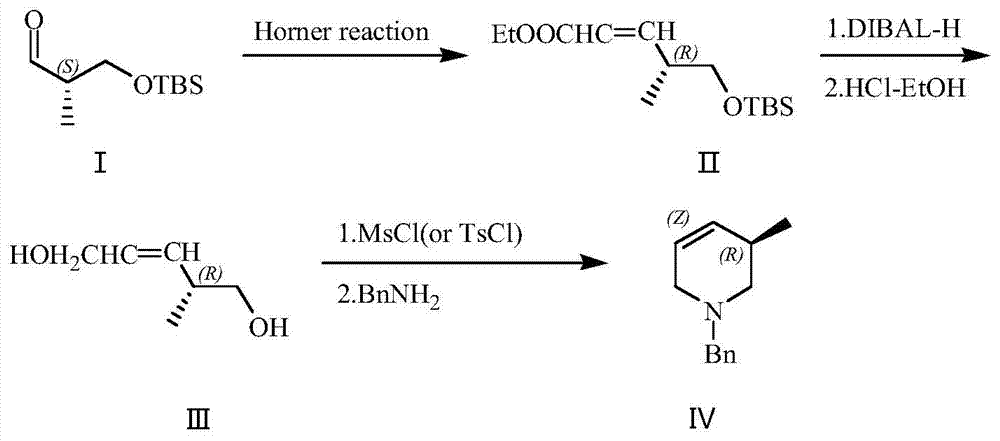
(R)-1-benzyl-3-methyl-1,2,3,6-tetrahydropiperidyl synthetic method
The invention provides a (R)-1-benzyl-3-methyl-1,2,3,6-tetrahydropiperidyl synthetic method, Which comprises the following steps: a)a substance shown in a formula I) is reacted to phosphate ester through Horner-Wadsworth-Emmons to generate a compound shown in a formula (II); b)the compound shown in the formula (II) is reduced by diisobutylaluminium hydride to obtain alcohol, and a deprotection reaction through concentrated hydrochloric acid-ethanol to obtain a compound shown in a formula (III); and c)the compound shown in the formula (III) is reacted through methylsulfonyl chloride or toluenesulfonyl chloride to obtain sulphonate or tosylate, and is performed with a benzylamine cyclization reaction in alcohol to obtain a compound shown in a formula (IV). The synthetic method is characterized in that initial raw materials, technical routes and post-treatment process are different, and the synthetic method has the advantages of simple and easily available raw materials, low cost and simple operation.
Owner:HANGZHOU ALLSINO CHEM
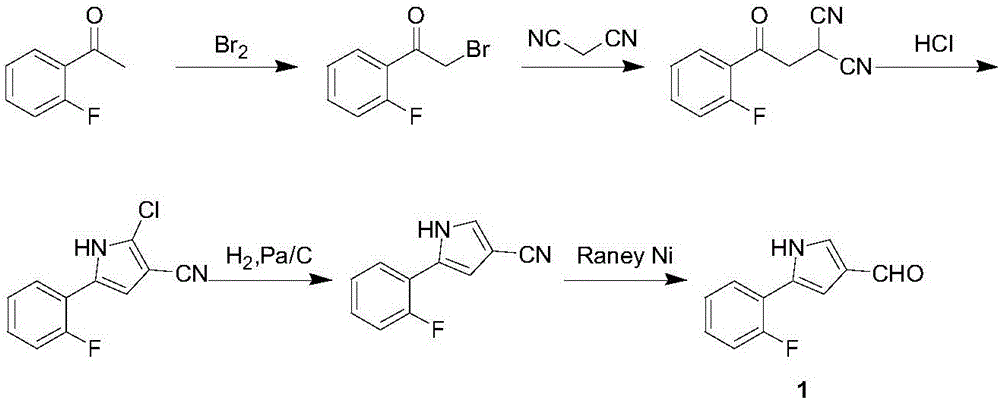
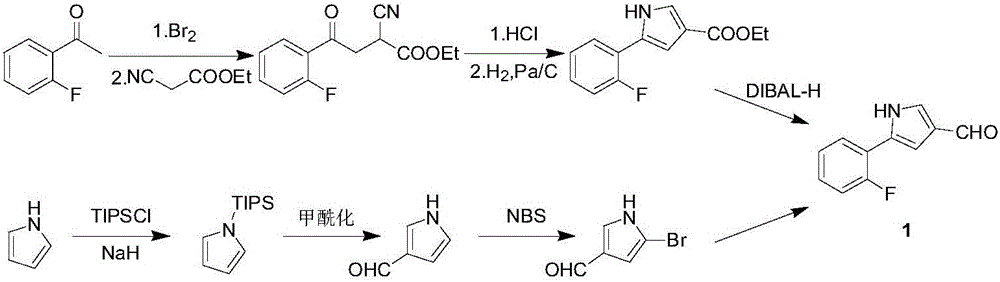
Preparation method of vonoprazan fumarate intermediate namely 5-(2-fluorophenyl)-1H-pyrrole-3-methanal
ActiveCN106243008AStable manufacturingQuality improvementOrganic chemistryChemical industryPalladium on carbon
The invention relates to a preparation method of a vonoprazan fumarate intermediate namely 5-(2-fluorophenyl)-1H-pyrrole-3-methanal, and belongs to the technical field of chemical industry. The preparation method comprises the following steps of (1) by using 2-fluoroacetophenone as an initial raw material, enabling the 2-fluoroacetophenone and allylamine to be subjected to condensation so as to obtain a compound IV; (2) under the catalysis of a metal catalyst, and under the condition that a ligand is provided, enabling the compound IV to be subjected to a ring-closing reaction so as to obtain a compound V; and (3) sequentially performing bromination, hydolysis and oxidation reactions by using the compound V so as to obtain the 5-(2-fluorophenyl)-1H-pyrrole-3-methanal. According to the preparation method disclosed by the invention, reagents of bromine, hydrogen chloride and the like, which have strong corrosivity, are prevented from being used, and flammable hydrogenation reducing agents of palladium on carbon, raneys nickel, diisobutylaluminium hydride and the like are also prevented from being used; and besides, the technological line is simple to operate, the reaction condition is mild, the product yield is high, the purity is high, and industrialized production is easy to realize.
Owner:SHANDONG JINCHENG BIO PHARMA CO LTD
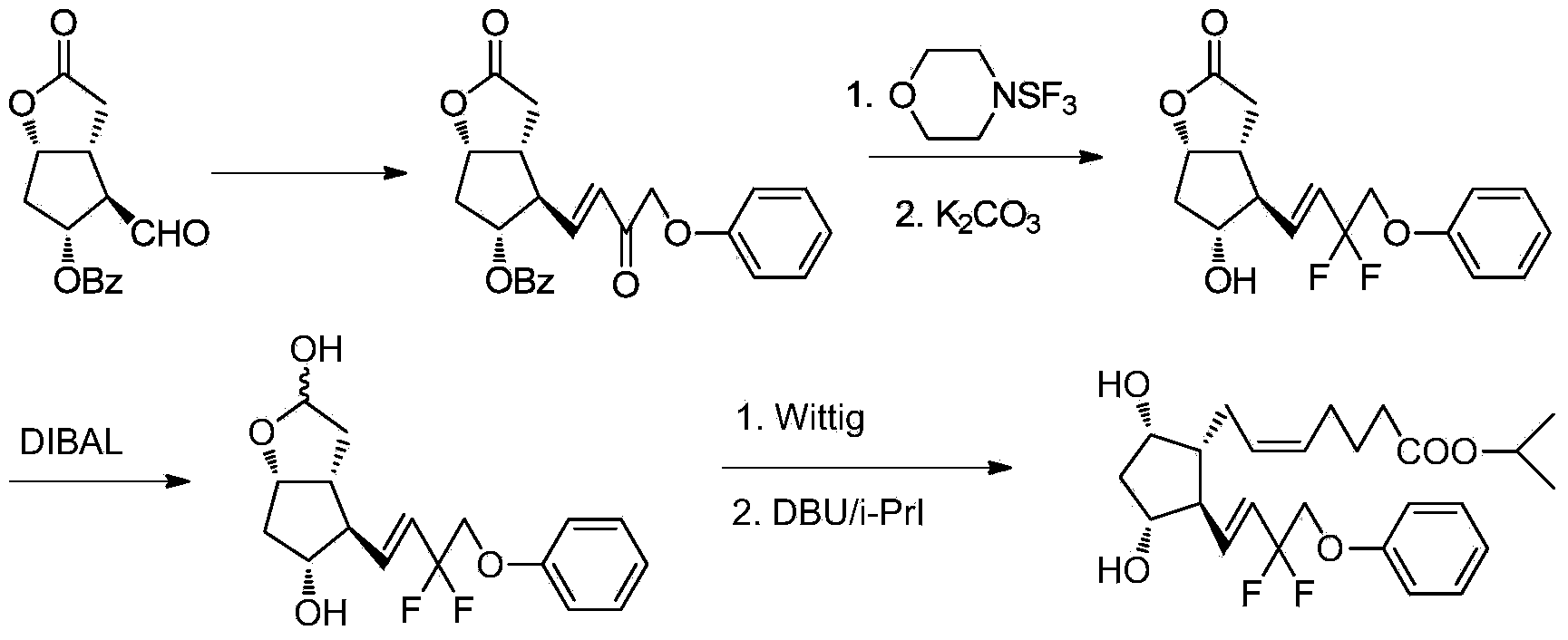
Novel method for synthetizing prostaglandin analogue
InactiveCN103288698AGroup 4/14 element organic compoundsBulk chemical productionWittig reactionKetone
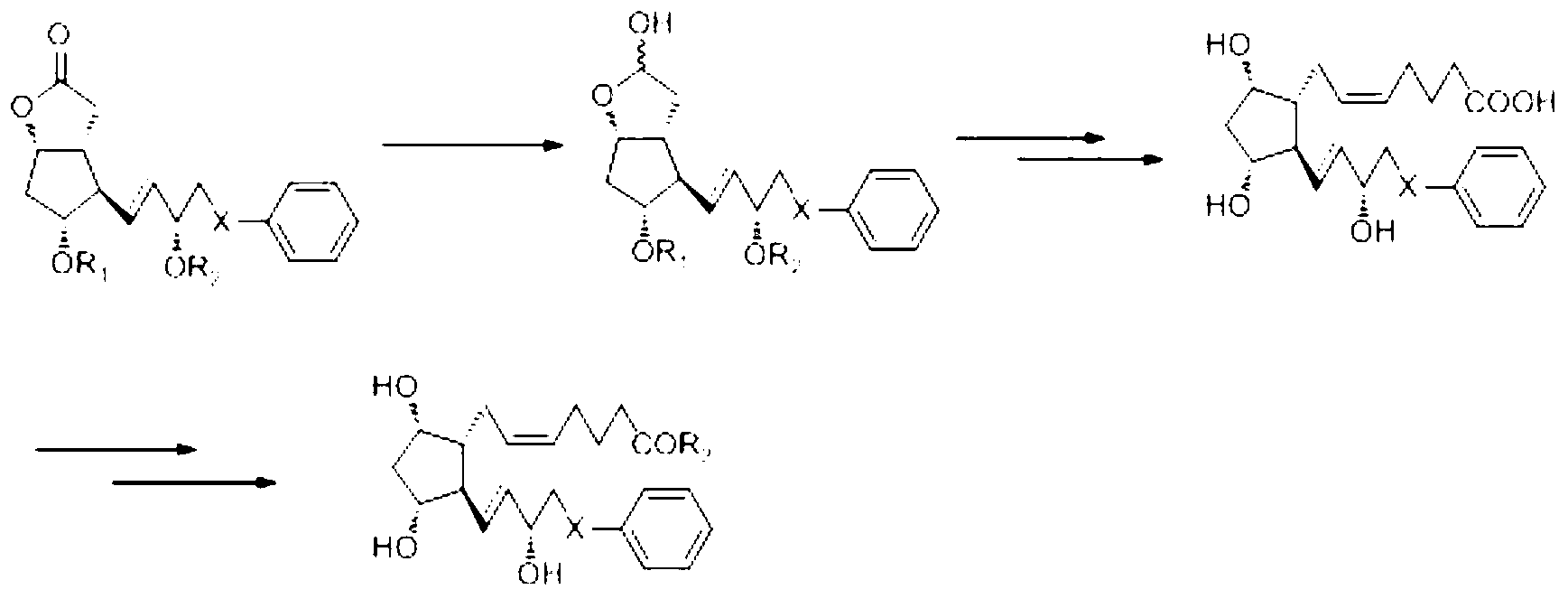
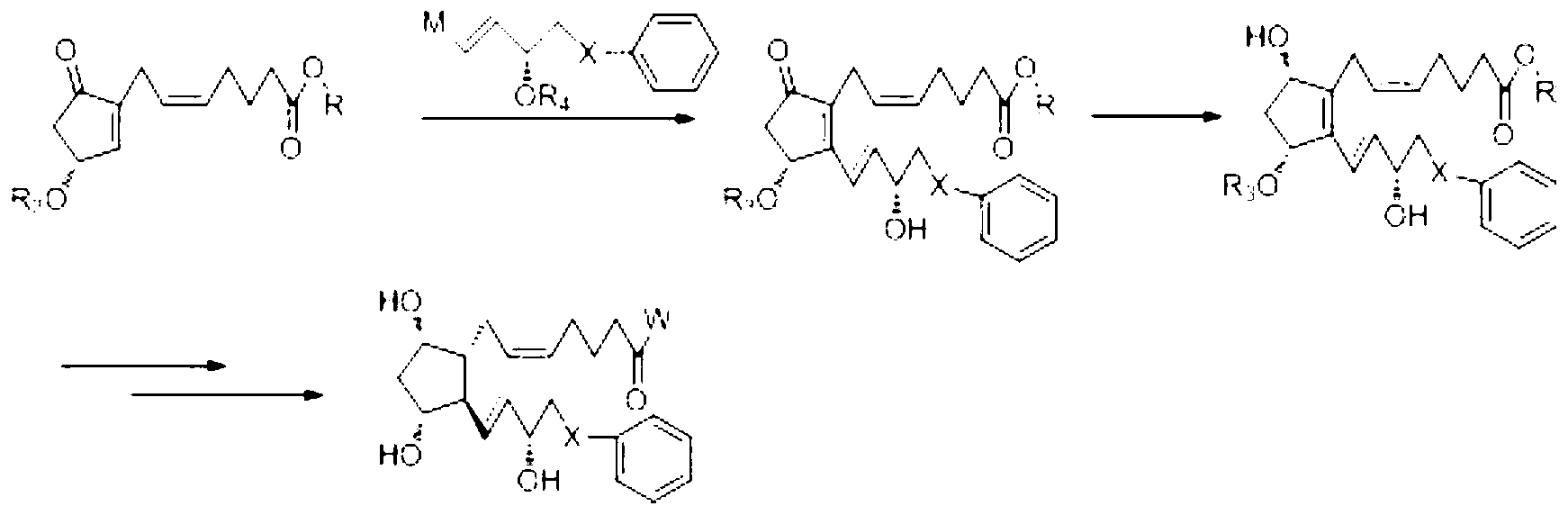
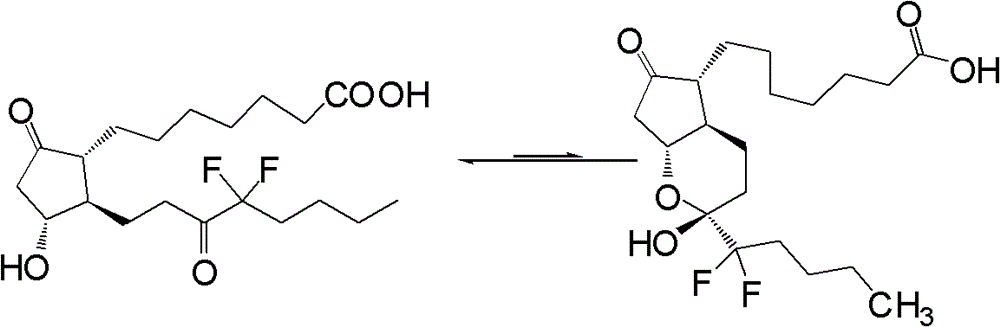
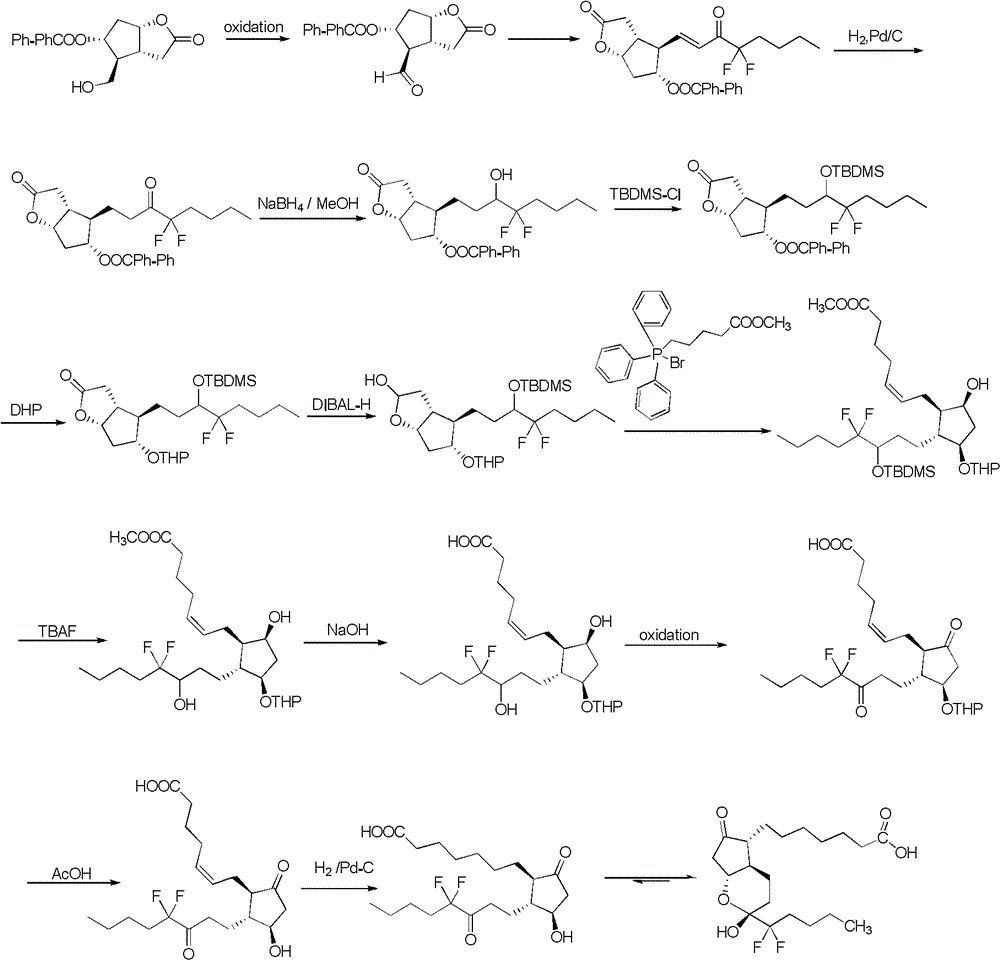
The invention relates to a novel method for synthetizing prostaglandin analogues, and an important intermediate compound related to the novel method. The method comprises the steps of (a), just protecting 15-site hydroxyl of the compound of a formula 2 by utilizing a hydroxyl protection group under an alkaline condition; (b) restoring a lactone ketone group from DIBAL (diisobutylaluminium hydride) to obtain the intermediate of a formula 4; (c) carrying out Wittig reaction on the intermediate of the formula 4 by utilizing 4-carboxy butyl-triphenylphosphine bromide, carrying out esterification or amidation after connecting an alpha chain; (d) removing 15-site hydroxyl protection group to obtain the prostaglandin analogue of the formula 1. The method is less in side reaction, easy to monitor, and convenient to separate due to the fact that the difference between a crude product and the polarity of a process material is large.
Owner:北京洛斯顿精细化工有限公司
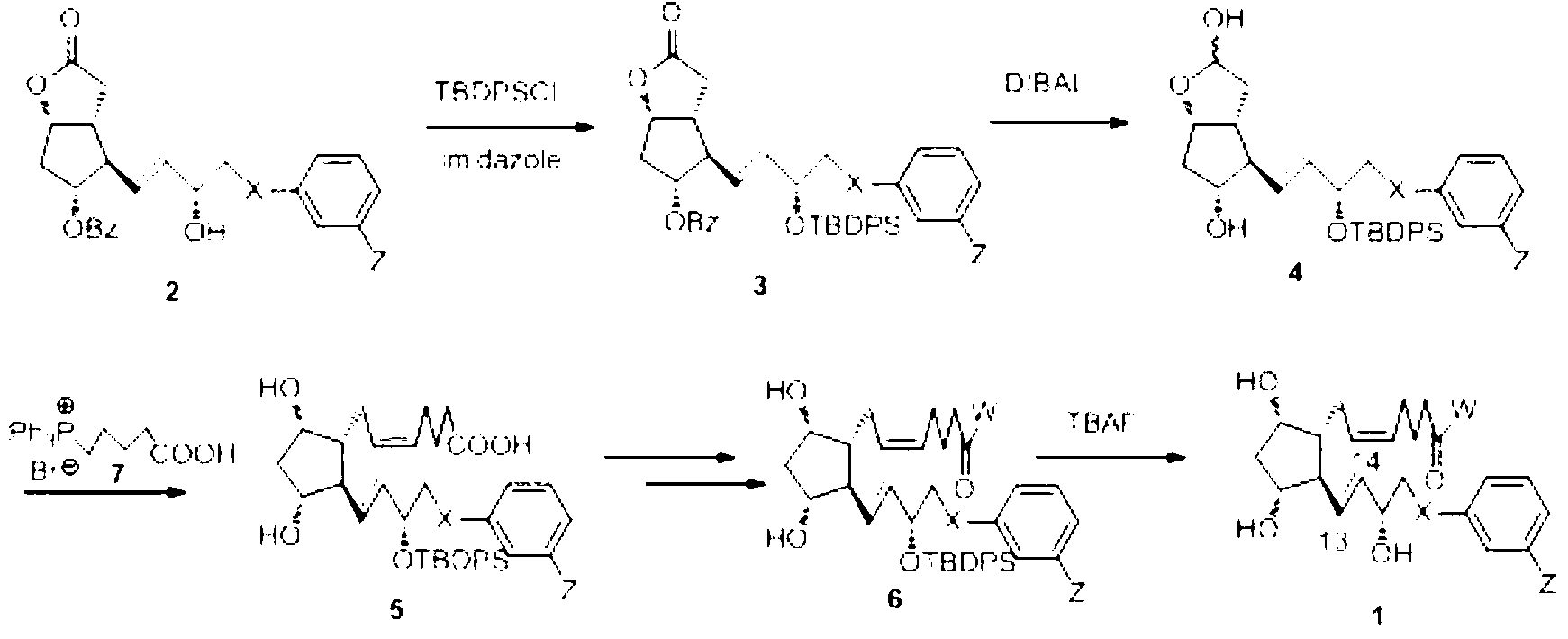
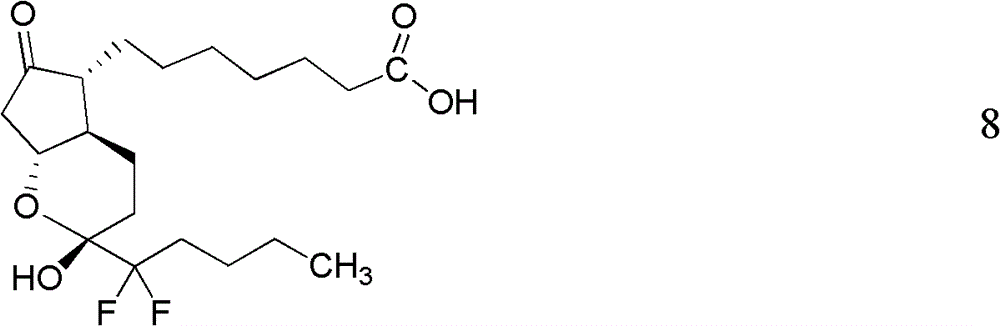
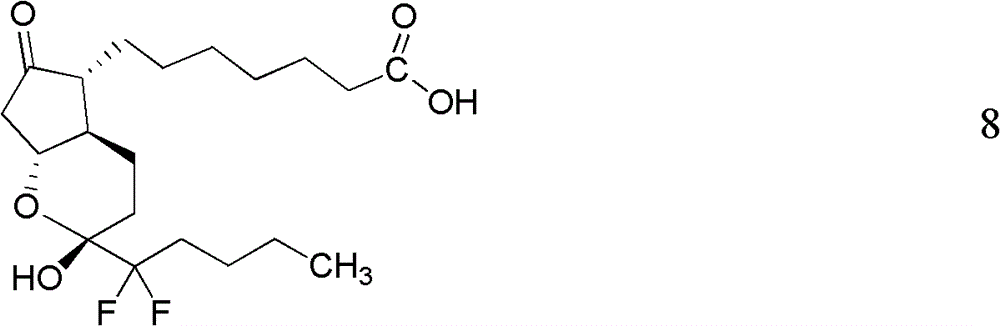
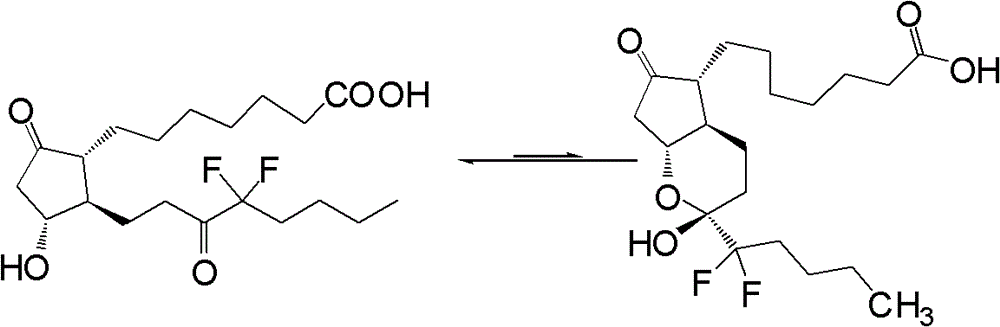
Preparation method of lubiprostone or midbody thereof
ActiveCN103058907AEasy to removeHigh yieldOrganic chemistryBulk chemical productionTert-butyldimethylsilaneWittig reaction
The invention discloses a novel method for preparing a lubiprostone midbody as shown in the formula 7. The method comprises the following steps: (1), a compound as shown in the formula 1 reacts with tert-butyldimethylsilyl chloride to selectively protect a primary hydroxyl group, thereby obtaining a compound shown in the formula 2; (2), a protecting group is applied to the compound 2 under the action of a catalyst, thereby obtaining a compound shown in the formula 3; (3), after the compound 3 is reduced through diisobutylaluminium hydride, a Wittig reaction is carried out on the compound 3, thereby obtaining carboxylic acid shown in the formula 4; (4), the compound 4 is protected in an acetonitrile solvent through a protecting group, thereby obtaining a compound shown in the formula 5; (5), the compound 5 is treated by using the tert-Butyldimethylsilane for removing the protecting group, thereby obtaining a compound shown in the formula 6; and (6), the compound 6 is oxidized by an oxidant and then reacts with a compound shown in the formula (10), thereby obtaining the higher-purity compound shown in the formula 7.
Owner:WUHAN QR PHARMA CO LTD
Process for the preparation of aldehydes
InactiveCN101720314AIncrease hardware investmentIncrease fluid pressureOrganic reductionPreparation by hydrogenolysisPhotochemistryDiisobutylaluminium hydride
The present invention is related to a method for the production of an aldehyde by reducing an ester of a carboxylic acid with H-DIBAL (diisobutylaluminium hydride).
Owner:LONZA LTD
Synthetic method for 4-bromo-6-chloronicotinaldehyde
ActiveCN109651241ASimple reaction conditionsMild reaction conditionsOrganic chemistryNitriteTrifluoroacetic acid
The invention provides a synthetic method for 4-bromo-6-chloronicotinaldehyde, and relates to the field of pharmaceutical chemistry. A synthetic route includes as follows: ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinate can be obtained through the reaction of ethyl 4,6-dichloronicotinate and 4-methoxybenzylamine; ethyl 4-amino-6-chloronicotinate can be obtained through the reaction of the ethyl 6-chloro-4-((4-methoxybenzyl)amino)nicotinate and trifluoroacetic acid; ethyl 4-bromo-6-chloronicotinate can be obtained through the reaction of the ethyl 4-amino-6-chloronicotinate and tert-butyl nitrite and benzyltriethylammonium bromide; (4-bromo-6-chloropyridin-3-yl)methanol can be obtained through the reaction of the ethyl 4-bromo-6-chloronicotinate and diisobutylaluminium hydride under a certain condition; and the (4-bromo-6-chloropyridin-3-yl)methanol can be further reacted under the synthetic action of manganese dioxide, so that a target product 4-bromo-6-chloronicotinaldehyde can beobtained. The disclosed synthetic method is mild in reaction condition, low in production cost and suitable for large-scale industrial production.
Owner:上海毕得医药科技股份有限公司
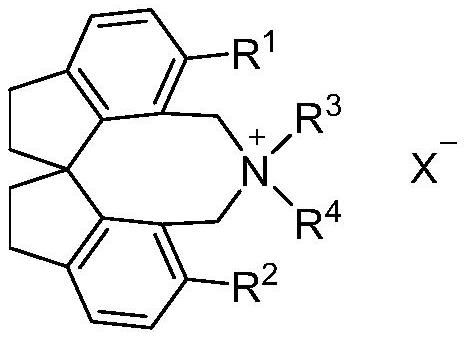
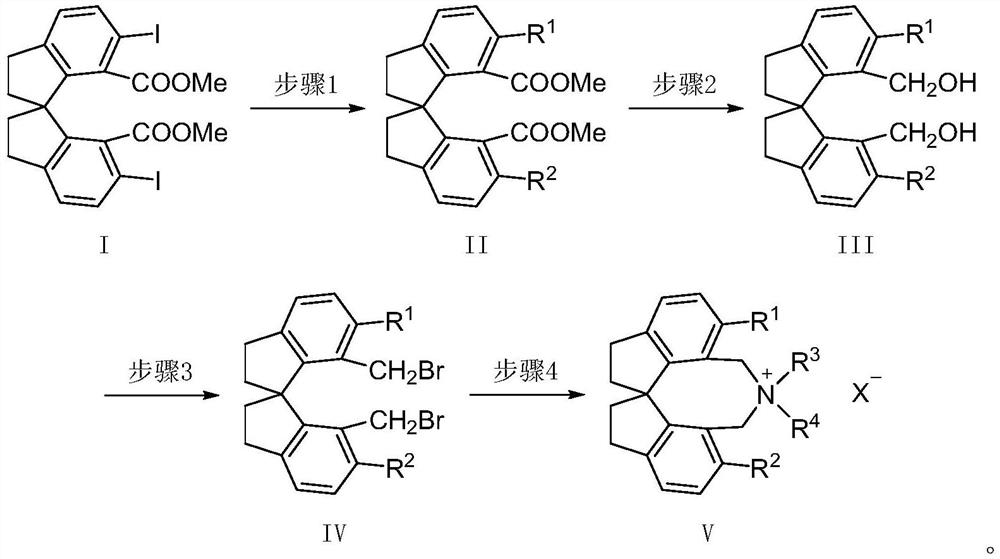
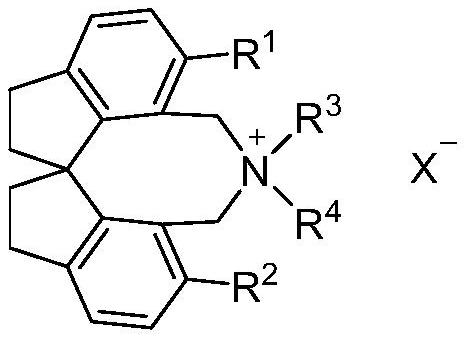
Spiroindane skeleton chiral quaternary ammonium salt as well as preparation method and application thereof
PendingCN114262295ARich varietyOrganic-compounds/hydrides/coordination-complexes catalystsImino compound preparationGlycinePhosphorus tribromide
The invention relates to spirobiindane skeleton chiral quaternary ammonium salt as well as a preparation method and application thereof. The preparation method comprises the following steps: under the action of bis (triphenylphosphine) palladium chloride and potassium carbonate, enabling optically active 6, 6 '-diiodo-1, 1'-spirobiindane-7, 7 '-dimethyl diformate to react with arylboronic acid to generate 6, 6'-disubstituted-1, 1 '-spirobiindane-7, 7'-dimethyl diformate; the preparation method comprises the following steps: reacting 6, 6 '-disubstituted-1, 1'-spirobiindane-7, 7 '-dimethanol with diisobutylaluminium hydride to generate 6, 6'-disubstituted-1, 1 '- The preparation method comprises the following steps: reacting 7, 7 '-disubstituted-7, 7'-bis (bromomethyl)-1, 1 '-spirobiindene with phosphorus tribromide to generate 6, 6'-disubstituted-7, 7 '-bis (bromomethyl)- And reacting with secondary amine under the action of potassium carbonate to generate corresponding chiral quaternary ammonium salt. The spirobiindane skeleton chiral quaternary ammonium salt can be used for catalyzing an asymmetric alkylation reaction of diphenylmethylene glycine tert-butyl ester and pentafluorobenzyl bromide, the highest yield of the obtained product can reach 98%, and the highest enantioselectivity can reach 95% ee.
Owner:TIANJIN UNIV
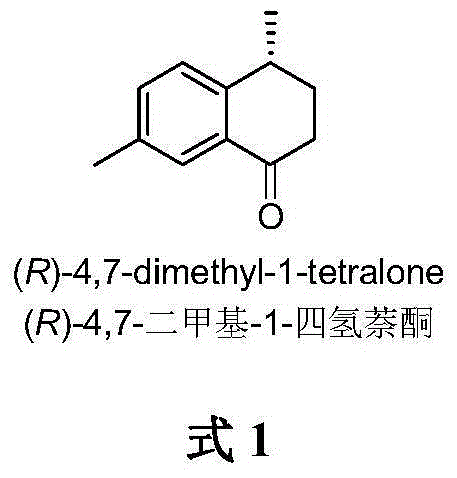
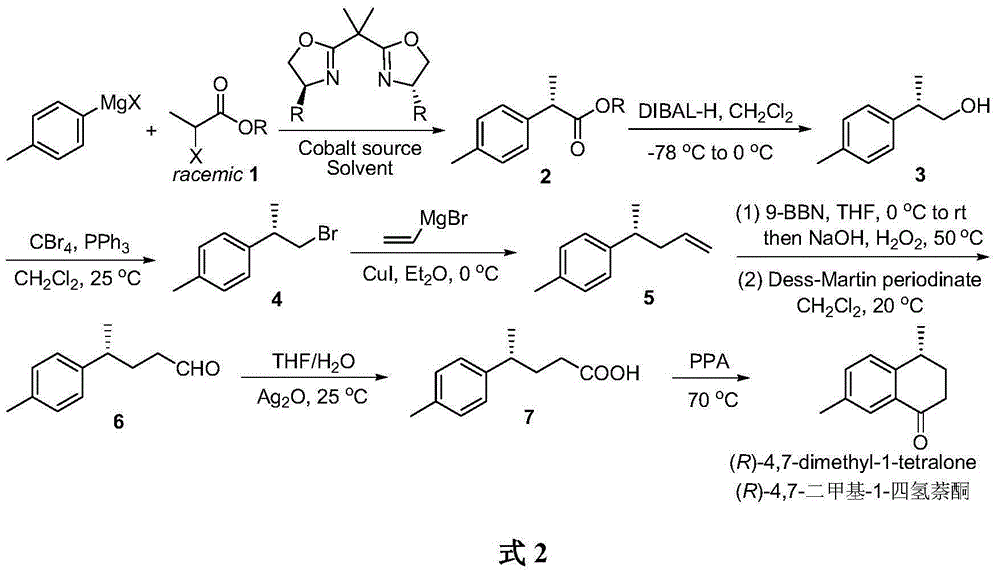
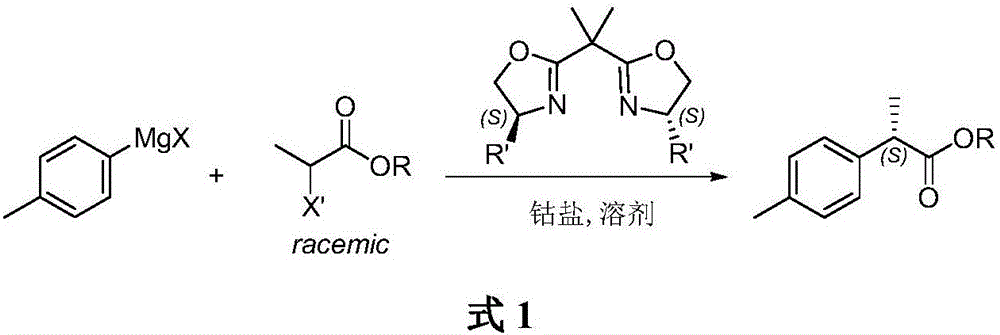
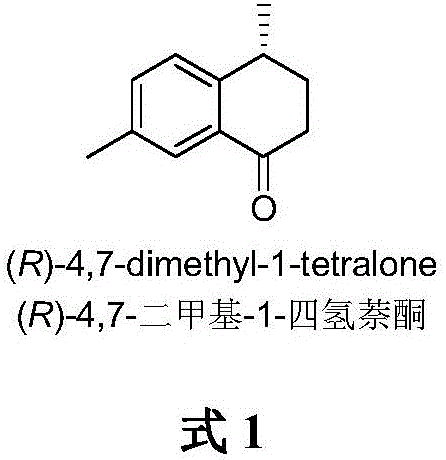
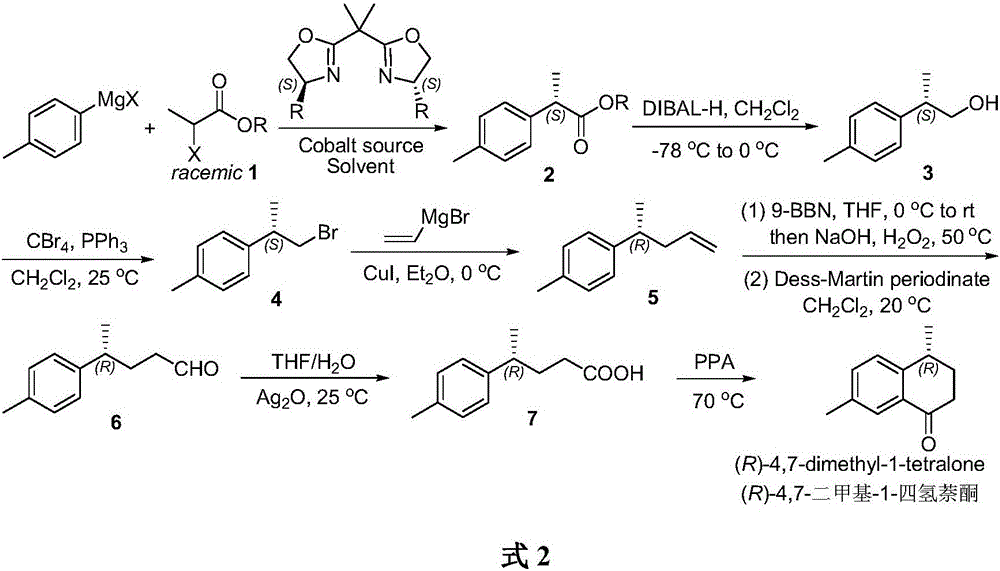
Method for asymmetrically catalyzing and synthesizing (R)-4, 7-dimethyl-1-tetralone
The invention discloses a method for asymmetrically catalyzing and synthesizing (R)-4, 7-dimethyl-1-tetralone. According to the method, an asymmetrical Kumada cross coupling reaction is conducted on racemization 2-halogenated propionate ester catalyzed by bis oxazoline / cobalt and a methyl phenyl grignard reagent, and (S)-P-toluene propionate ester 2 is firstly generated; then, (S)-P-toluene propionate ester 2 is reduced to (S)-P-toluene propyl alcohol 3 through diisobutylaluminium hydride (DIBAL-H), and then (R)-4-p-methylphenyl-1-amylene 5 is obtained through bromine generation and coupling with and vinyl grignard reagent; next, a hydroboration-oxidation reaction and a Dess-Martin oxidizing reaction are sequentially conducted, and (R)-4-p-methylphenyl valeraldehyde 6 is obtained; finally, oxidation is conducted through silver oxide, an intramolecular Fourier acyl reaction is conducted, and (R)-4,7-dimethyl-1-tetralone is obtained in a ring-closure synthesis mode. The synthesis route is simple and concise, 8 reactions are conducted in all, the total yield is 27%, and the optical purity of a product is 90%.
Owner:CHINA AGRI UNIV
SiAlCN(O) ceramic nano fiber, and preparation method and application thereof
InactiveCN108911759AEasy to operateSimple processNitrogen and non-metal compoundsFiberElectrospinning
The invention discloses an SiAlCN(O) ceramic nano fiber, and a preparation method and application thereof. The preparation method comprises the following steps: (1) preparation of polyaluminium silazane; (2) preparation of SiAlCN(O) nano fibers; and (3) preparation of the SiAlCN(O) ceramic nano fibers. According to the SiAlCN(O) ceramic nano fiber provided by the invention, the polyaluminium silazane synthesized by using a toluene solution of methyl vinyl dichlorosilane, methylhydrodichlorosilane and diisobutylaluminium hydride is taken as a raw material, and the nano ceramic fiber is obtainedthrough a high-temperature pyrolysis method after electrostatic spinning. The technological operation of the preparation method provided by the invention is simple, the sintering temperature is low,energy consumption is reduced, and cost is saved.
Owner:中科广化(重庆)新材料研究院有限公司 +3
Preparation method of bis (1, 5-cyclooctadiene) nickel
The invention discloses a preparation method of bis (1, 5-cyclooctadiene) nickel, and the method comprises the following steps: (1) adding 1, 5-cyclooctadiene into 0.5-1.0 M of anhydrous nickel diacetylacetonate tetrahydrofuran solution under anhydrous and anaerobic conditions, cooling to -78 DEG C, then dropwise adding an organic solution of diisobutyl aluminum hydride into the tetrahydrofuran solution, heating to -10 to 25 DEG C after the addition, and reacting for 1-5 hours; (2) performing filter pressing under the protection of an inert gas to obtain bis (1,5-cyclooctadiene) nickel crystals, and then preserving the bis (1,5-cyclooctadiene) nickel crystals in the atmosphere of the inert gas and avoiding light. The method provided by the invention has mild reaction conditions for preparing the bis (1,5-cyclooctadiene) nickel, is convenient for industrial batch production, and has high product purity and good yield.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
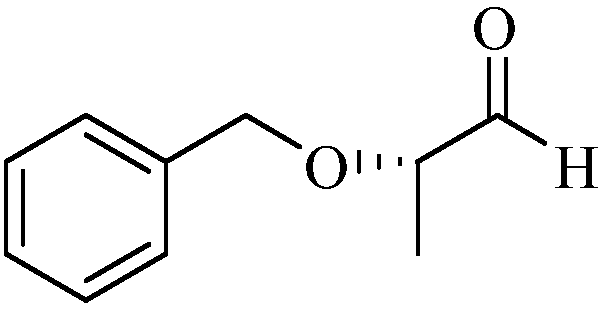
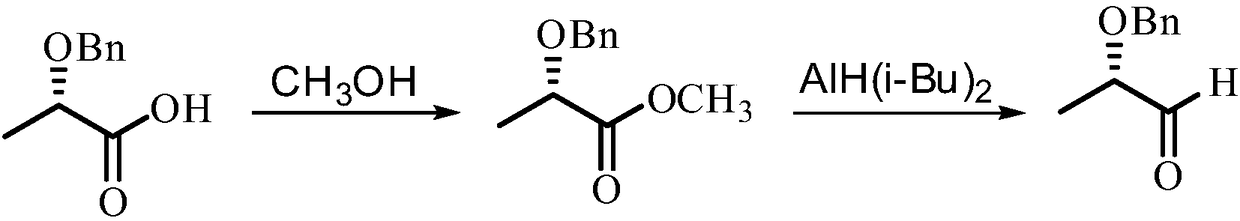
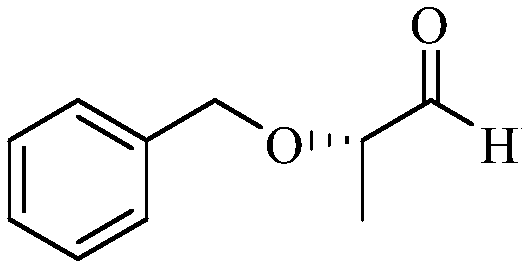
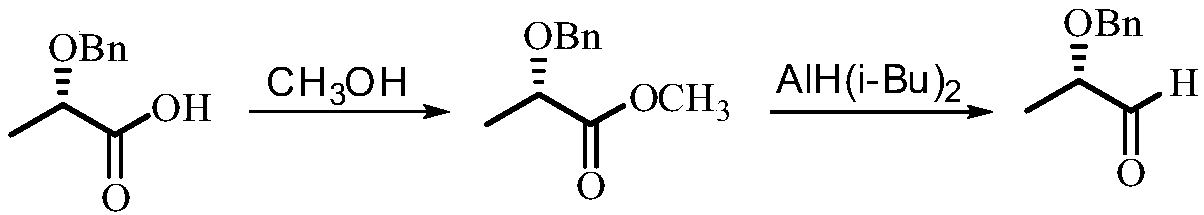
Method for synthesizing (S)-2-benzyloxy-1-propanal
ActiveCN108101759AImprove operational safetyReduce manufacturing costOrganic compound preparationOrganic chemistry methodsEthylenediamineSolvent
The invention discloses a method for synthesizing (S)-2-benzyloxy-1-propanal. The method comprises the following steps: firstly, enabling (S)-2-benzyloxy-1-propanal to react with ethylenediamine, andobtaining (S)-2-(1-benzoxy ethyl)-4,5-dihydrogen-1H-imidazole; dissolving the (S)-2-(1-benzoxy ethyl)-4,5-dihydrogen-1H-imidazol in anhydrous ethanol, then adding metal sodium under the protection ofnitrogen, and stirring and reacting; and after the reaction is ended, removing the ethanol, slowing adding residues into a saturated oxalic acid solution, uniformly stirring, then refluxing reacting,after the reaction is ended, extract, drying and filtering, removing the solvent, and obtaining a product (S)-2-benzyloxy-1-propanal. The (S)-2-benzyloxy-1-propanal is prepared in two steps; in the preparation process, diisobutylaluminium hydride which is expensive in price and easy to ignite in the after-treatment is not used, so that the operation safety is improved, and the production cost is decreased; and the preparation process is mild and easy in operation, the anhydrous condition for the reaction of a traditional process is not, and the industrialized production is easy to realize.
Owner:东营睿港投资服务有限责任公司
Synthesis of strigolactone (±)-gr24 and 4-substituted (±)-gr24
ActiveCN106518822BSimple and fast operationImprove securityOrganic chemistryBenzoic acidStrigolactone
Owner:SHAANXI NORMAL UNIV
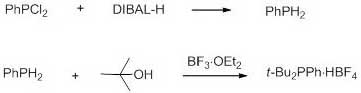
A method for synthesizing di-tert-butylphenylphosphonium tetrafluoroborate
ActiveCN109438511BReduce usageSimplify the experimental stepsGroup 5/15 element organic compoundsDichlorophenylphosphineTetrafluoroborate
The invention discloses a method for synthesizing di-tert-butylphenylphosphonium tetrafluoroborate, and belongs to the field of organic synthesis. The method comprises the following steps: in the anhydrous and anaerobic atmospheres, taking dichlorophenylphosphine as a raw material, after the reduction of diisobutyl aluminium hydride, reacting with tertiary butanol under the catalytic action of boron trifluoride, and then carrying out hydrolysis to generate the di-tert-butylphenylphosphonium tetrafluoroborate. Compared with the prior art, the method is high in yield and simple in aftertreatment, and is more applicable for industrial production.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
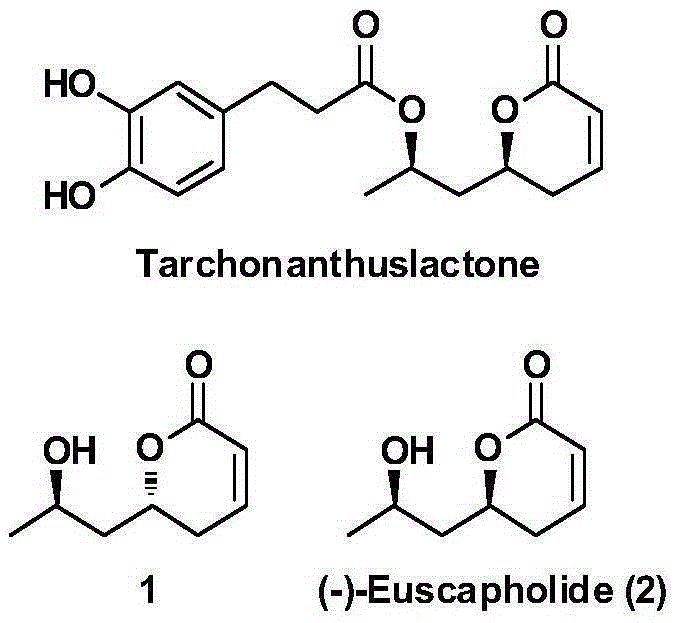
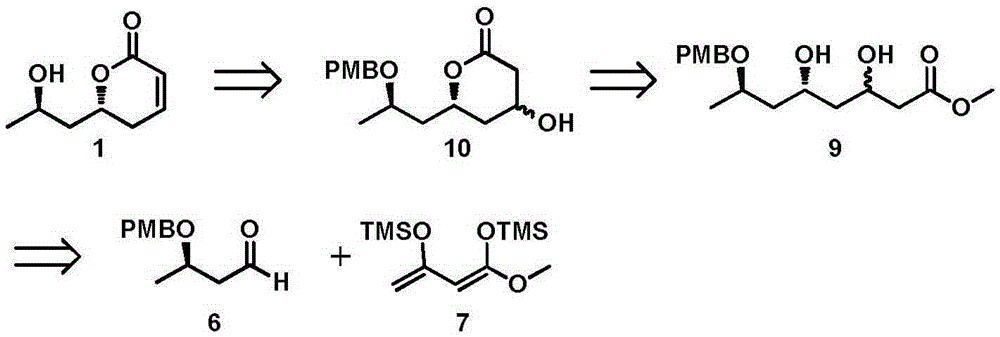
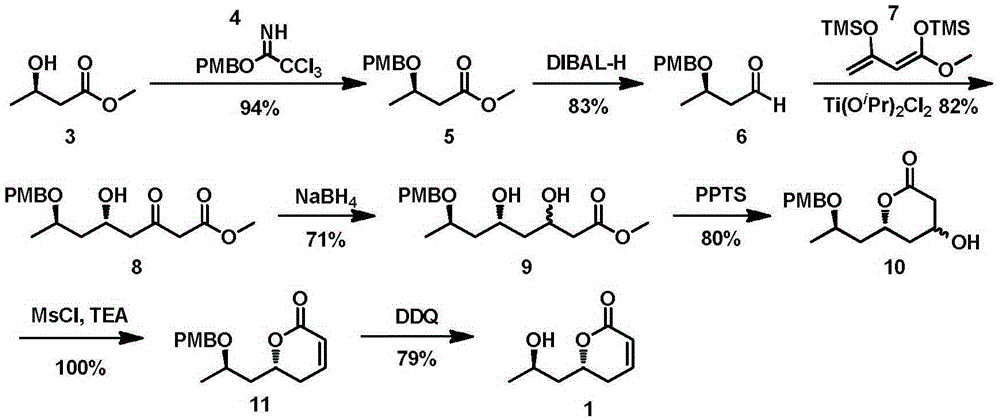
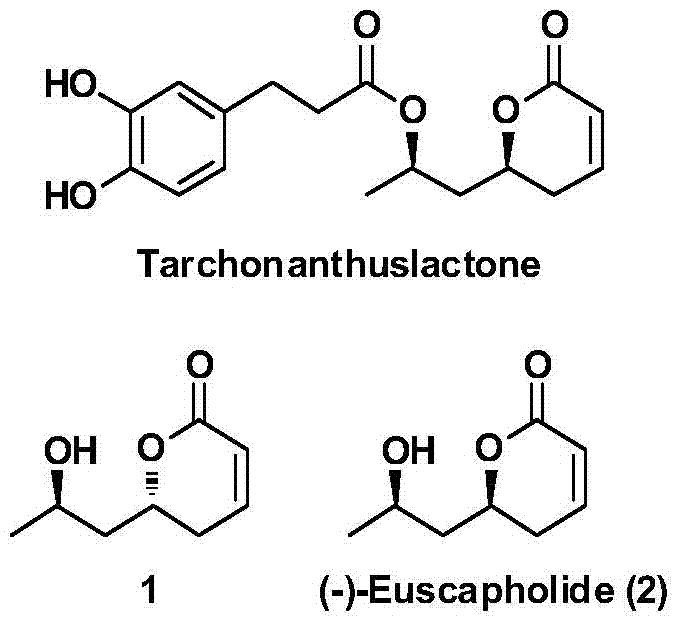
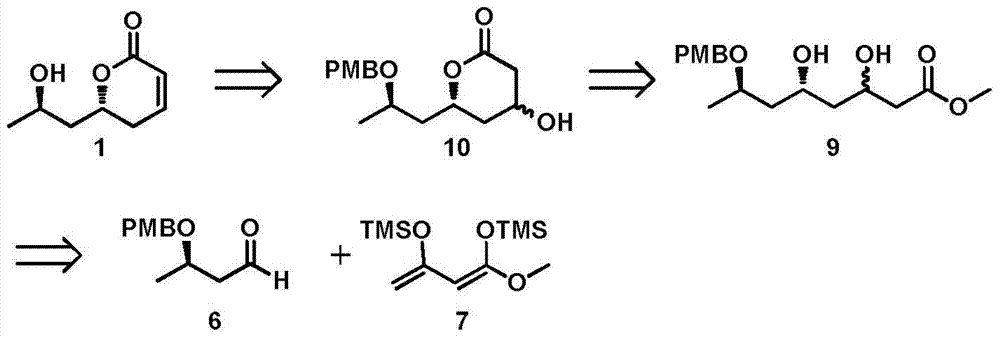
New method for asymmetrically synthesizing natural product (-)-Euscapholide isomer
InactiveCN105399714ANovel and reasonable designRaw materials are easy to getOrganic chemistry methodsBulk chemical productionButyrateNatural product
The invention relates to a new method for asymmetrically synthesizing a natural product (-)-Euscapholide isomer. The method comprises the following steps: carrying out methoxybenzyl group protection on (R)-3-hydroxymethyl butyrate used as an initial raw material, reducing by using diisobutylaluminium hydride, carrying out a Mukaiyama aldol reaction, reducing by using sodium borohydride, carrying out ring closure under acidic conditions, eliminating hydroxyl groups, and deprotecting to complete asymmetric total synthesis of the target molecule 1. The method has the advantages of novel and reasonable synthesis route design, cheap and easily available raw material, simple operating process, mild reaction conditions, efficient completion of the asymmetric total synthesis of the Euscapholide isomer with two chiral centers, and single product configuration.
Owner:JIANGXI SCI & TECH NORMAL UNIV
A new method for the asymmetric synthesis of isomers of the natural product (‑)‑euscapholide
InactiveCN105399714BNovel and reasonable designRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionHydroxymethylPhotochemistry
The present invention relates to a novel method for asymmetrically synthesizing natural product (-)-Euscapholide isomers. Starting with (R)‑3‑hydroxybutyrate methyl ester, after p-methoxybenzyl protection, diisobutylaluminum hydride reduction, Mukaiyama aldol reaction, sodium borohydride reduction, acidic ring closure, hydroxyl Elimination, deprotection and other processes complete the asymmetric total synthesis of the target molecule 1. The synthetic route design is novel and reasonable, the raw materials are cheap and easy to obtain, the operation process is simple, the reaction conditions are mild, and the asymmetric total synthesis of one isomer of Euscapholide with two chiral centers is efficiently completed, and the product configuration is single.
Owner:JIANGXI SCI & TECH NORMAL UNIV
A kind of method of asymmetric catalytic synthesis (r)-4,7-dimethyl-1-tetralone
The invention discloses a method for asymmetrically catalyzing and synthesizing (R)-4, 7-dimethyl-1-tetralone. According to the method, an asymmetrical Kumada cross coupling reaction is conducted on racemization 2-halogenated propionate ester catalyzed by bis oxazoline / cobalt and a methyl phenyl grignard reagent, and (S)-P-toluene propionate ester 2 is firstly generated; then, (S)-P-toluene propionate ester 2 is reduced to (S)-P-toluene propyl alcohol 3 through diisobutylaluminium hydride (DIBAL-H), and then (R)-4-p-methylphenyl-1-amylene 5 is obtained through bromine generation and coupling with and vinyl grignard reagent; next, a hydroboration-oxidation reaction and a Dess-Martin oxidizing reaction are sequentially conducted, and (R)-4-p-methylphenyl valeraldehyde 6 is obtained; finally, oxidation is conducted through silver oxide, an intramolecular Fourier acyl reaction is conducted, and (R)-4,7-dimethyl-1-tetralone is obtained in a ring-closure synthesis mode. The synthesis route is simple and concise, 8 reactions are conducted in all, the total yield is 27%, and the optical purity of a product is 90%.
Owner:CHINA AGRI UNIV
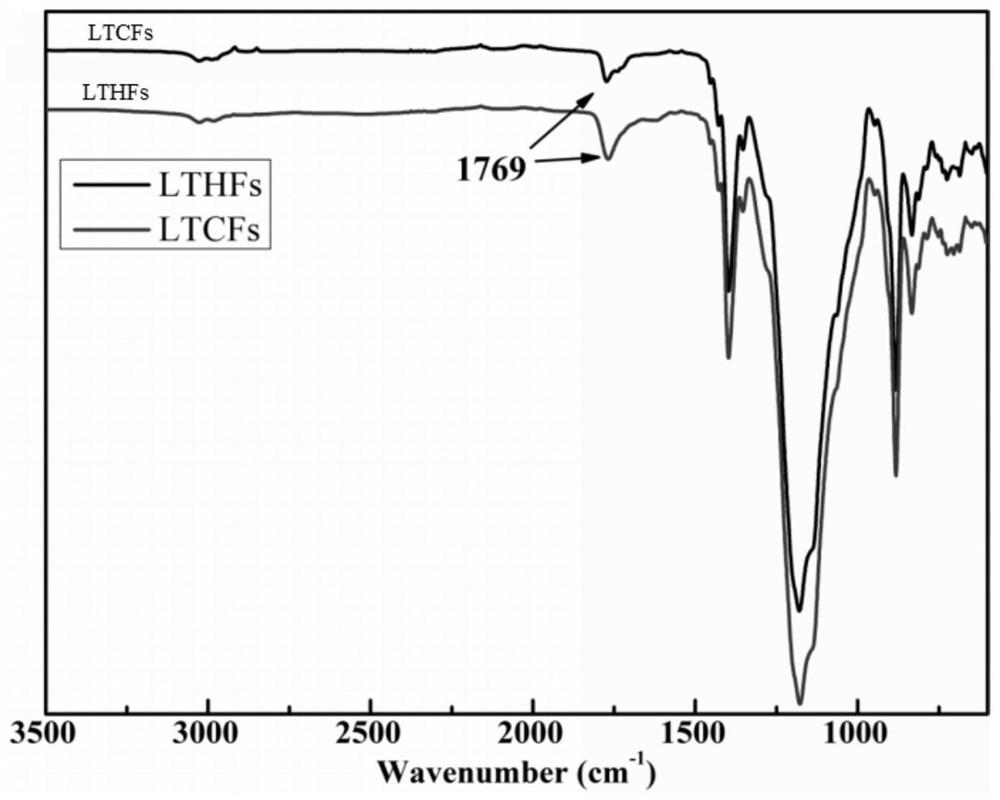
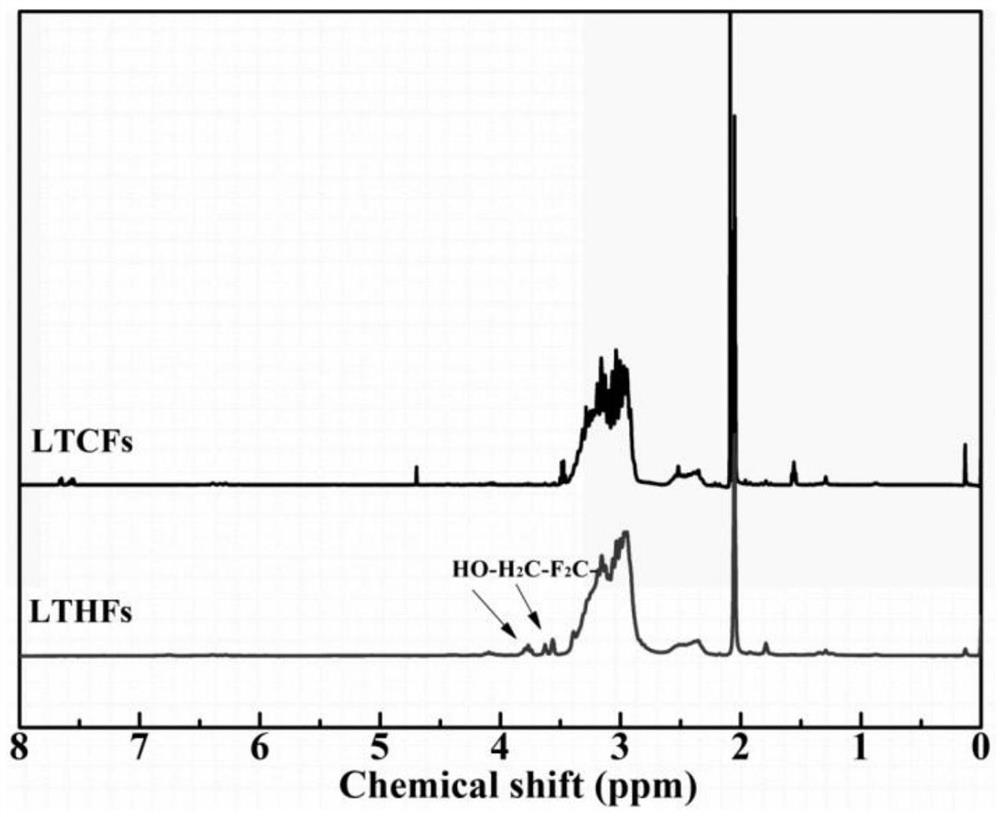
Method for reducing low-molecular-weight carboxyl-terminated fluorine-containing polymer by hydroboron/diisobutylaluminium hydride reduction system
The invention discloses a method for reducing a low-molecular-weight carboxyl-terminated fluorine-containing polymer by using a hydroboron / diisobutylaluminium hydride reduction system. According to the method, hydroboron and diisobutylaluminium hydride are compounded to form a reduction system for reducing the low-molecular-weight carboxyl-terminated fluorine-containing polymer, the method has the characteristics of small reducing agent dosage, safe reaction, simple post-treatment process, good selectivity, better reduction effect of a composite system than that of a single system and the like, and the reduction rate is up to 80% or above. The product can be applied as a functional fluorine-containing polymer intermediate, an adhesive, a joint mixture, a coating, a processing compounding agent and the like.
Owner:DALIAN MARITIME UNIVERSITY
Preparation method of lubiprostone or its intermediate
ActiveCN103058907BNot suitable for storageHigh yieldOrganic chemistryBulk chemical productionTert-butyldimethylsilaneWittig reaction
Owner:WUHAN QR PHARMA CO LTD
Photosensitizer probe TFDB as well as preparation method and application thereof
ActiveCN111606937AEfficient singlet oxygen generation capabilityImprove photostabilityPhotodynamic therapyGroup 3/13 element organic compoundsSinglet oxygenALUMINUM HYDRIDE
The invention discloses a photosensitizer probe TFDB as well as a preparation method and application thereof. The preparation method comprises the following steps: dissolving pentafluorobenzonitrile in a solvent, stirring for reaction, dropwise adding ammonia water in an ice bath, and treating a reaction product to obtain an intermediate 1; dissolving the intermediate 1 in ultra-dry dichloromethane in a nitrogen atmosphere, slowly adding diisobutyl aluminum hydride at-78 DEG C, stirring for reaction at-78 DEG C, and treating a reaction product to obtain an intermediate 2; dissolving the intermediate 2, 2, 4-dimethylpyrrole and trifluoroacetic acid in a solvent, stirring for reaction, adding 2, 3-dichloro-5, 6-dicyano-1, 4-benzoquinone, quickly adding triethylamine and a boron trifluoride diethyl ether complex in an ice bath, and treating areaction product to obtain an intermediate 3; and dissolving the intermediate 3 and N-iodosuccinimide in dichloromethane, and treating areaction product to obtain the photosensitizer probe TFDB. The photosensitive probe TFDB shows efficient singlet oxygen generation capacity and fluorescence emission capacity, and has the potential of being used for real-time monitoring of tumor photodynamic therapy and fluorescence imaging.
Owner:NANJING UNIV OF TECH
Efficient synthesis method of histamine H3 receptor antagonist pitolisant
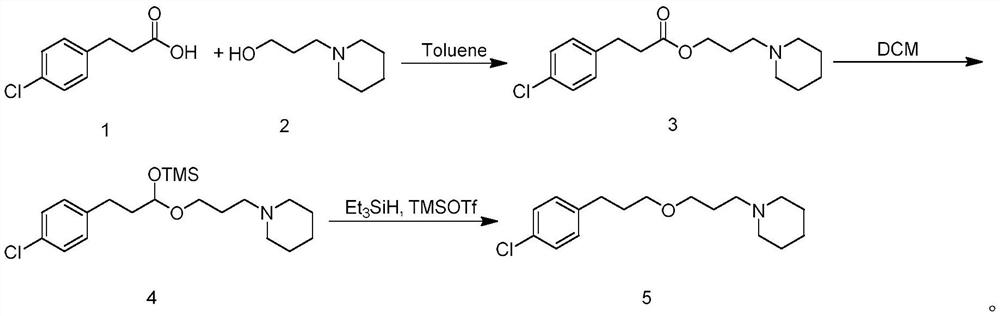
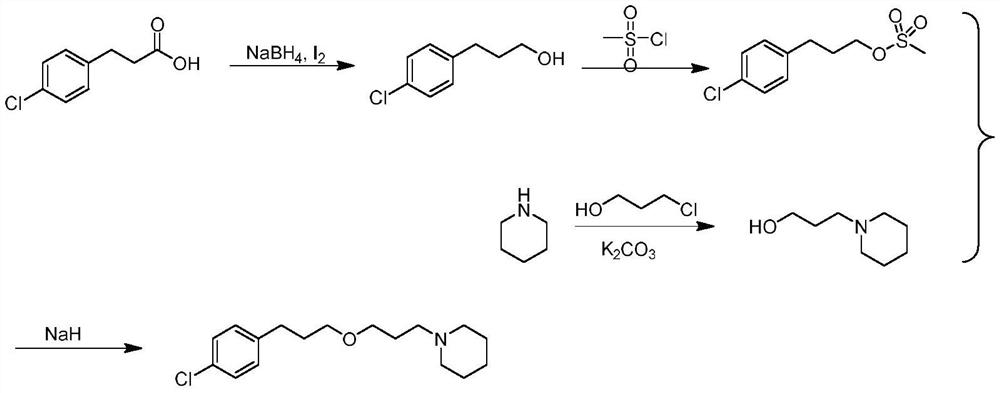
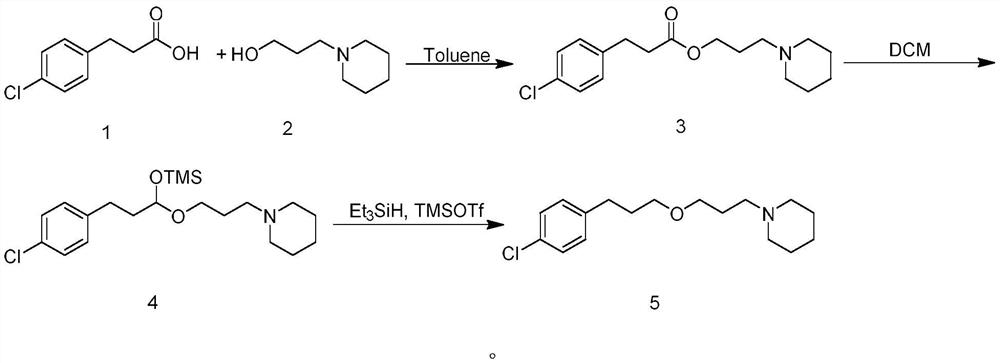
ActiveCN114014825ASolve bottlenecksShort reaction pathOrganic chemistryBulk chemical productionPropanoic acidChlorobenzene
The invention discloses an efficient synthesis method of histamine H3 receptor antagonist pitolisant, which comprises the following specific steps: directly carrying out esterification reaction on 3-(4-chlorphenyl) propionic acid serving as an initial raw material and 1-piperidine propanol under the catalytic action of acid to obtain a compound 3; reducing the compound 3 into a compound 4 under the action of a reduction system, with the reduction system being a mixed system of trimethylsilylimidazole and diisobutylaluminium hydride or a mixed system of trimethylsilylimidazole and red aluminum; and reducing the compound 4 under the combined action of a mixed system of triethyl silane and trimethylsilyl trifluoromethanesulfonate to obtain the target product pitolisant. The method has the advantages of short reaction route, high production efficiency and simple and easily available raw materials.
Owner:HENAN NORMAL UNIV
Synthetic method of (S)-2-benzyloxy propionaldehyde
InactiveCN109651132AImprove operational safetyReduce manufacturing costOrganic compound preparationOrganic chemistry methodsPropanoic acidHydrogen atmosphere
Disclosed is a synthetic method of (S)-2-benzyloxy propionaldehyde. The synthetic method includes the following concrete steps: (1) firstly, (S)-2-benzyloxy propionic acid with an acylation reagent toobtain (S)-2-benzyloxy propionyl chloride; and (2) adding a palladium on barium sulfate catalyst into o-xylene, subjecting the palladium on barium sulfate catalyst and the o-xylene to a reduction reaction for 15-30 minutes, adding the (S)-2-benzyloxy propionyl chloride prepared in the step (1), continuing to heat for reflux reaction in a hydrogen atmosphere until the reaction mixture does not absorb hydrogen, filtering off the catalyst after the reaction is completed, and removing the o-xylene to obtain the product (S)-2-benzyloxy propionaldehyde. Instead of using diisobutylaluminium hydride,which is prone to ignition after treatment, as a reaction raw material, the acylation reagent which is low in cost, safer and more environmentally friendly and the palladium on barium sulfate catalyst which can be recycled for many times are used as reaction raw materials, the reaction process is more consistent with the principle of atomic economy, and the reaction is more mild.
Owner:NINGBO XINKAI BIOTECH CO LTD
Preparation method of ultrahigh cis-polybutadiene rubber
The invention relates to the technical field of functional materials and particularly relates to a preparation method of ultrahigh cis-polybutadiene rubber. The preparation method of the ultrahigh cis-polybutadiene rubber comprises the following steps: (1) carrying out nitrogen displacement on a baked and dried ampoule bottle for several times, filling the ampoule bottle with nitrogen, quantitatively injecting an Nd hexane solution, a Bd hexane solution and a diisobutylaluminium hydride hexane solution into the ampoule bottle respectively by virtue of a disposable syringe, carrying out binary ageing on mixed liquid in the ampoule bottle, measuring out a diisobutyl aluminum chloride hexane solution by virtue of the disposable syringe, injecting the diisobutyl aluminum chloride hexane solution into binary ageing liquid, and carrying out ternary ageing; and (2) sequentially adding the Bd hexane solution and an Nd-series catalyst into a 50mL ampoule bottle which is subjected to vacuum drying and nitrogen displacement, and terminating polymerization reaction, so as to prepare the ultrahigh cis-polybutadiene rubber. The cis-1,4-structural molar fraction of polybutadiene rubber prepared by virtue of the preparation method exceeds 99%, and the maximum can reach 100%. The method is simple in operation and can be easily promoted in a large scale.
Owner:SHAANXI JUJIEHAN CHEM CO LTD
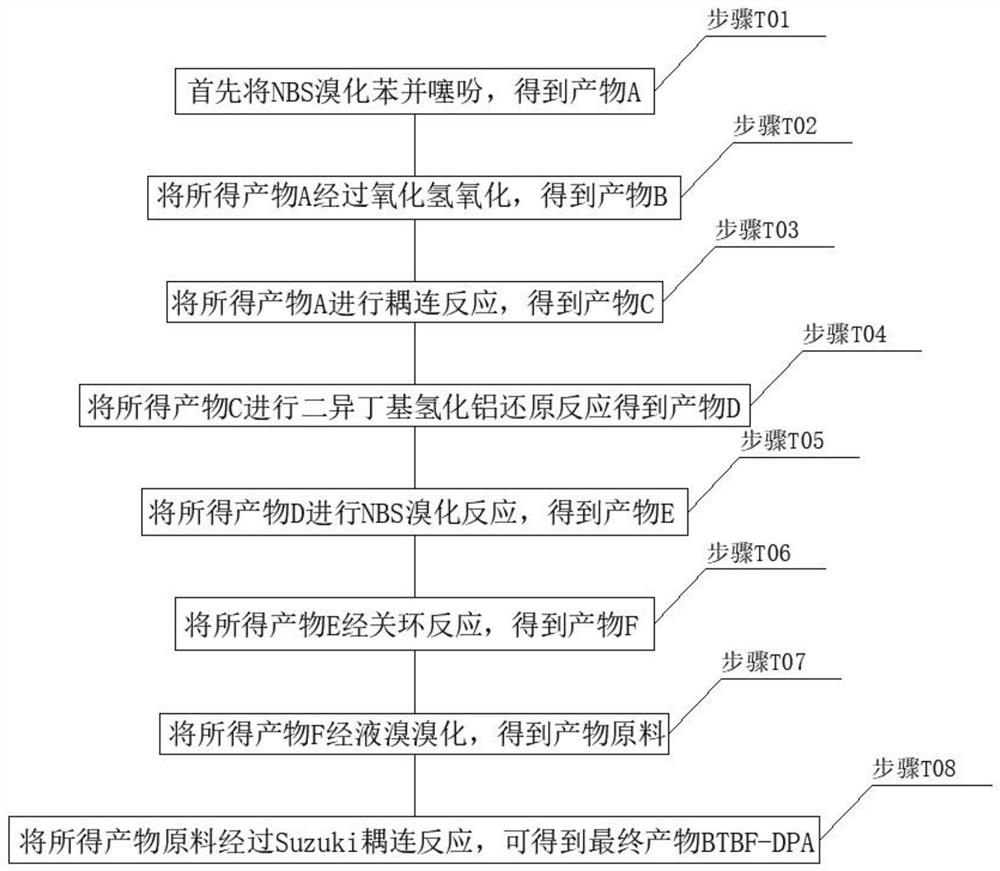
Preparation method and application of NPB-like hole transport functional material
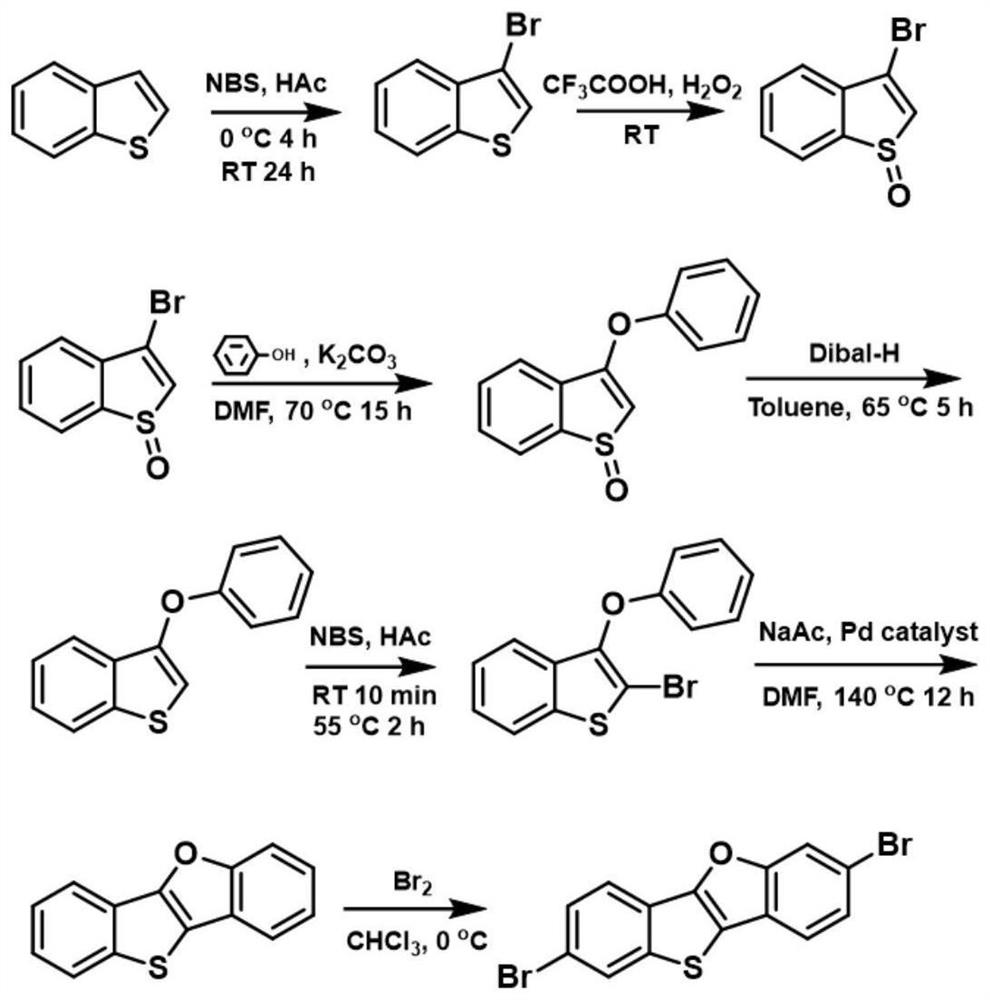
PendingCN113248517ANegativeHigh practical valueOrganic chemistryElectrography/magnetographyBenzothiopheneCombinatorial chemistry
The invention belongs to the technical field of organic photosensitive drums, and provides a preparation method and application of an NPB-like hole transport functional material. The NPB-like hole transport functional material comprises BTBF-DPA. The BTBF-DPA is used for an electronegative organic photoconductor drum of a laser printer. The preparation method comprises the following steps: T01, brominating benzothiophene with NBS to obtain a product A; T02, oxidizing the obtained product A with hydrogen peroxide to obtain a product B; T03, subjecting the obtained product A to a coupling reaction to obtain a product C; T04, subjecting the obtained product C to a diisobutylaluminium hydride reduction reaction to obtain a product D; T05, subjecting the obtained product D to an NBS bromination reaction to obtain a product E; T06: carrying out a ring closing reaction on the obtained product E to obtain a product F; T07, brominating the obtained product F through liquid bromine to obtain a product raw material; and T08: carrying out a Suzuki coupling reaction on the obtained product raw material to obtain the final product BTBF-DPA. The preparation method has the advantages of strict hierarchy, no need for sequential coating of a photosensitive drum structure, simple technical process, lower cost and high practical value.
Owner:GUANGDONG LEPUTAI NEW MATERIAL TECH
Synthesis method of key intermediate of immunomodulator
PendingCN113493423ALess metal residueOrganic compound preparationPreparation by hydrogenolysisHydroxylamineHydrogen Sulfate
The invention provides a synthesis method of a key intermediate of an immunomodulator, which comprises the following steps: dissolving a compound 2 and a compound 3, adding potassium carbonate, replacing nitrogen, and adding S-Phos to react; cooling, filtering, concentrating, and washing and drying an organic phase to obtain a compound 4; dissolving in tetrahydrofuran, and adding N, O-dimethyl hydroxylamine hydrochloride and triethylamine; stirring at room temperature, adding dichloromethane after quenching, and washing with an ammonium chloride aqueous solution; drying an organic phase, concentrating by using tetrahydrofuran, adding diisobutylaluminium hydride into a tetrahydrofuran solution for reaction, adding a potassium hydrogen sulfate aqueous solution, filtering an obtained mixture, and adding dichloromethane and hydrochloric acid for washing; concentrating an organic phase to be dry to obtain a compound 5; mixing with a compound 6 to react in dichloromethane, adding a Dess-Martin oxidation reagent, and purifying to obtain a compound 1; according to the route, only one step of metal catalysis is used, and the step is far away from a final compound, so that metal residues in medicine molecules are greatly reduced.
Owner:SUZHOU IBIO TECH CO LTD
Polyacene conductive agent and lithium ion battery by using polyacene conductive agent
ActiveCN104505514AImprove performanceImprove cycle performanceCell electrodesSecondary cellsAluminium chlorideNitrogen gas
The invention relates to the technical field of lithium ion battery, and concretely relates to a polyacene conductive agent and a lithium ion battery by using the polyacene conductive agent. The conductive agent is prepared by the following steps: under nitrogen atmosphere, in a sodium hydroxide solution with mass concentration of 3-5wt%, 1-3mol of ortho-aminophenol and 0.005-0.01mol of lithium aluminium hydride are reacted for 3-5 hours at 40-60 DEG C, then 1-2mol of benzene and 0.01-0.02mol of diisobutylaluminium hydride are added and reacted for 3-5 hours at 50-70 DEG C, then 0.1-0.2mol of anhydrous aluminium chloride is reacted for 1-2 hours at 30-35 DEG C, hydrochloric acid is used for neutralization after reaction is completed, dilute hydrochloric acid is used for cleaning, then sintering is carried out, after ball milling, secondary sintering is carried out to obtain the polyacene conductive agent. The polyacene conductive agent has the advantages of high conductivity, easy dispersion, good thermostabilization and environmental protection.
Owner:NINGBO VEKEN BATTERY
Novel method of synthesizing tafluprost
ActiveCN103804195AOrganic compound preparationCarboxylic acid esters preparationWittig reactionDiisobutylaluminum hydride
The invention relates to a novel method of synthesizing tafluprost. The method comprises the following steps: after suitable protection by Corey Lactone, reducing by DIBAL (Diisobutylaluminium hydride); carrying out Wittig reaction, carboxyl protection and Swern oxidization to obtain a universal intermediate compound 1; and by taking the intermediate 1 as a raw material, carrying out reactions to obtain tafluprost (deprotection step is not taken into consideration, and fluorination is the last step) and a plurality of intermediate products. The intermediate 1 is connected with different omega chains to synthesize other various prost analogues such as misoprost, travoprost and latanoprost. 2-4 prost analogues can be further used for synthesizing other 16-phenoxyl prost.
Owner:TIEN TIANJIN PHARMA
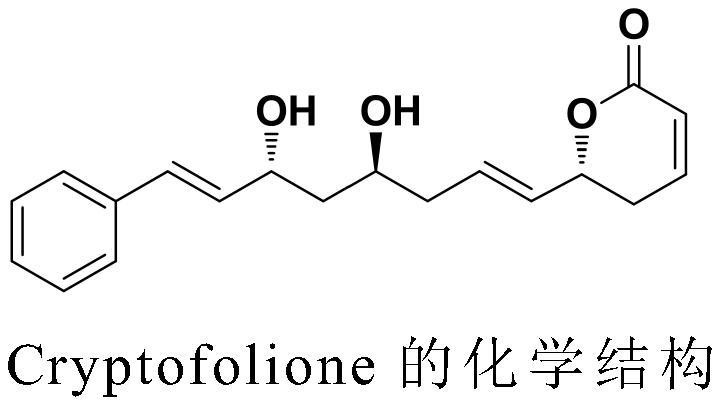
A kind of synthesis method of anti-trypanosome, anti-cancer natural product cryptofolione
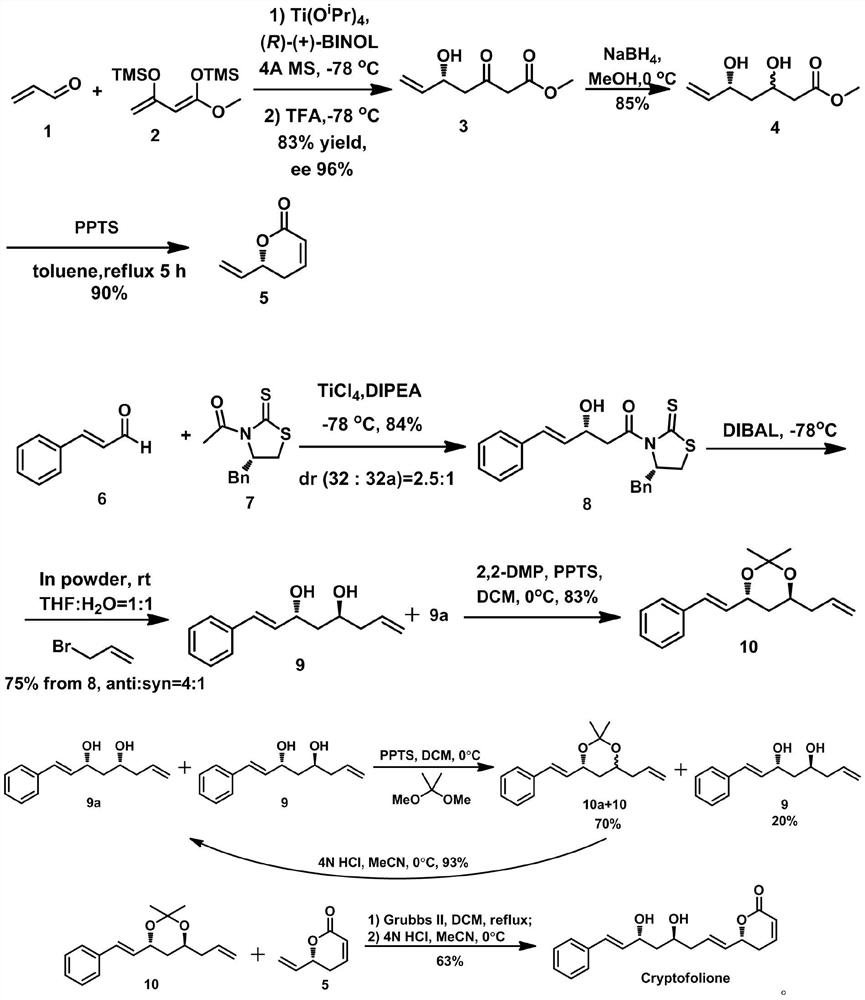
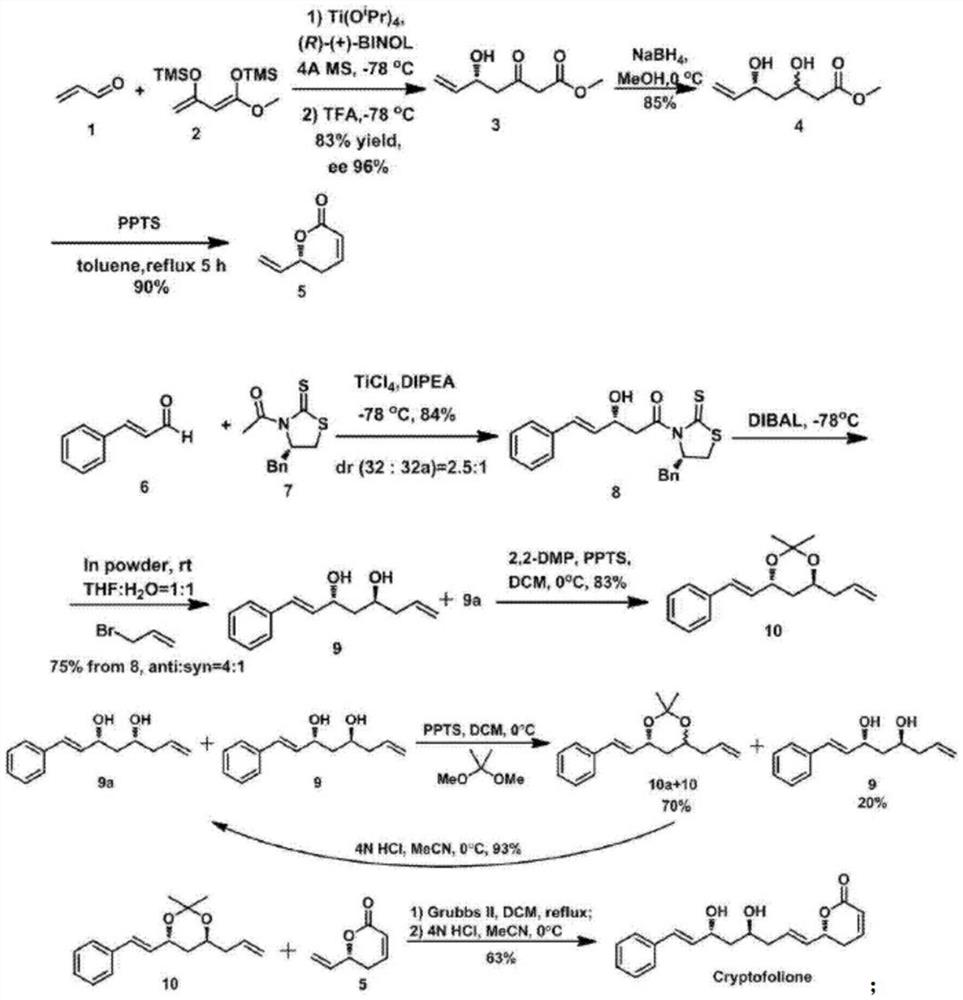
ActiveCN108484554BSolve the problem of chiral constructionSimple and fast operationOrganic chemistryBulk chemical productionKetoneALUMINUM HYDRIDE
The invention discloses an anti-trypanosome anticancer natural product Cryptofolione synthesizing method. The synthesizing method disclosed by the invention comprises the steps: after acrolein and silyl enol ether are utilized to perform Mukaiyama aldol reaction, sodium borohydride is used for reducing ketone into alcohol, and then pyridinium p-toluenesulfonate refluxes in a methylbenzene solutionto obtain compound shown in a formula 5; after cinnamyl aldehyde and an evans chiral aid are used for performing evans aldol reaction, diisobutyl aluminum hydride is used for reducing the cinnamyl aldehyde and the evans chiral aid at first, and then addition with metal indium activated 3-propylene bromine is performed to obtain compound shown in a formula 9; the compound shown in the formula 9 isresolved and purified for many times by kinetic resolution; the compound shown in the formula 9 and 2,2-diemthoxy propane can obtain fragment type 10 compound under the pyridinium p-toluenesulfonatecondition, the fragment type 10 compound and fragment type 5 compound can generate olefin metathesis reaction through a Grubbs secondary catalyst, and protecting groups of the fragment type 10 compound are removed under the hydrochloric acid condition to obtain Cryptofolione.
Owner:DONGGUAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com