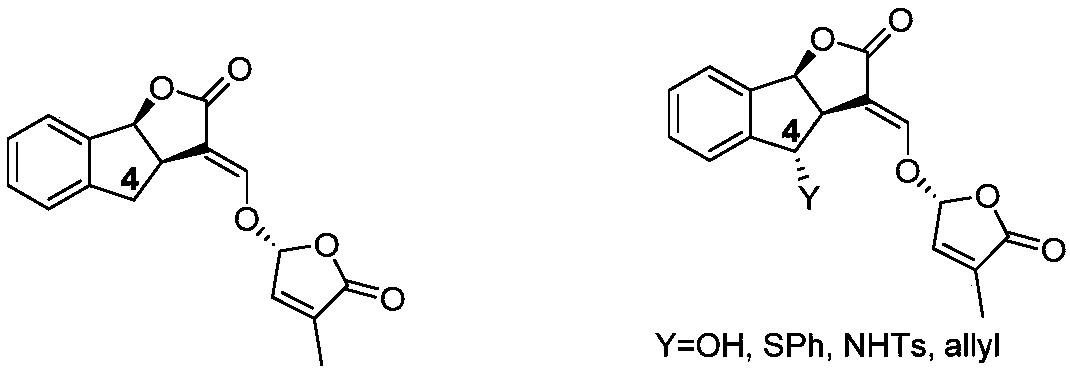
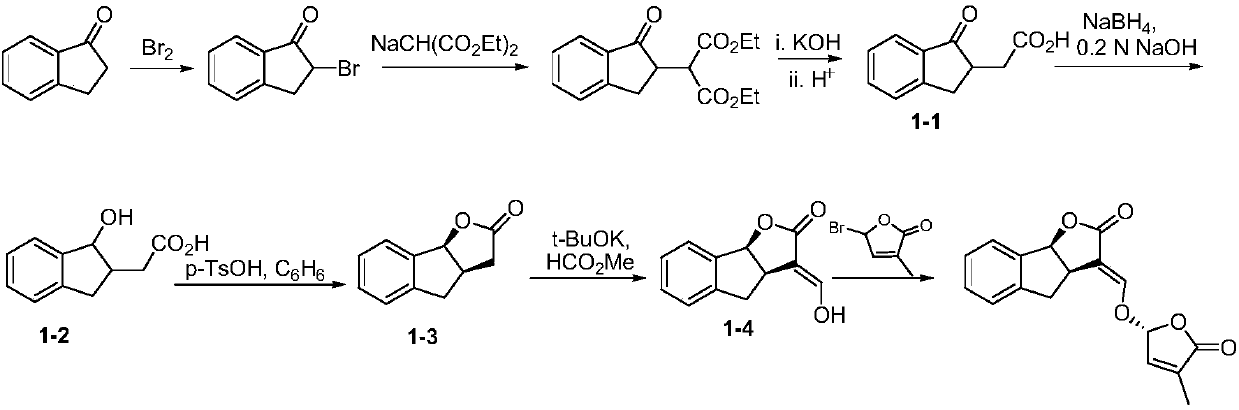
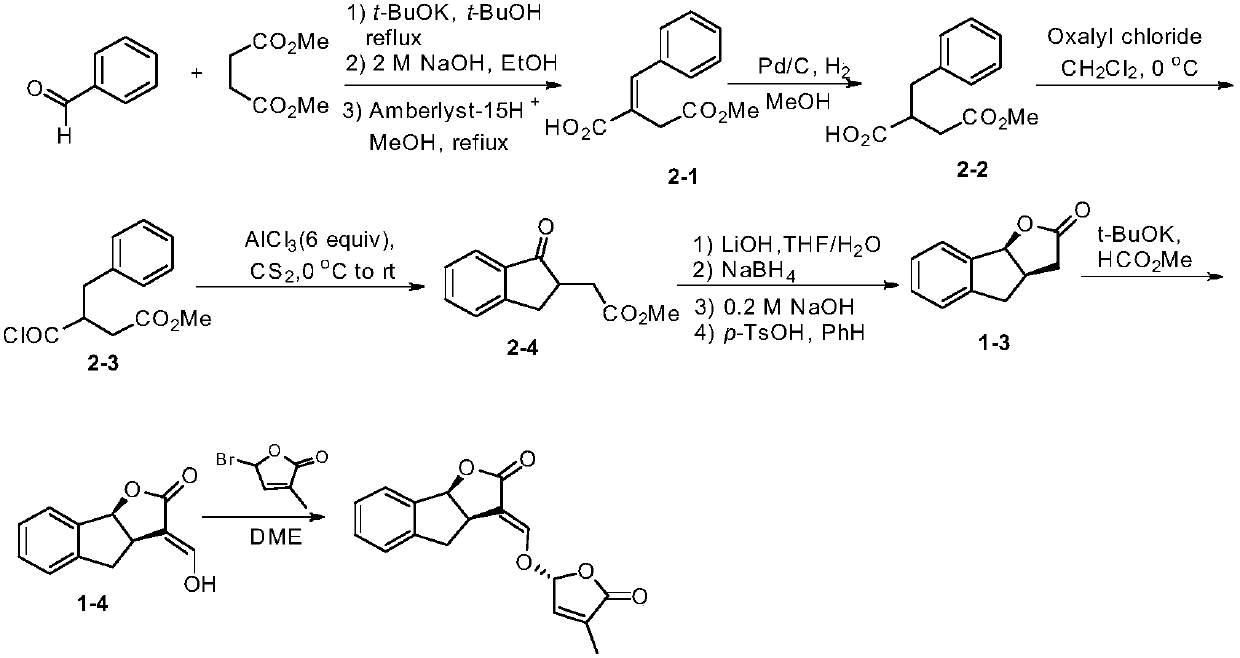
Synthesis of strigolactone (±)-gr24 and 4-substituted (±)-gr24
One-GR24, strigolactone technology, applied in the field of strigolactone synthesis, can solve the problems of environmental pollution, large amount of catalyst, long reaction steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Taking the synthesis of (±)-GR24 as an example, the synthesis method is as follows:
[0037] 1. Dissolve 1.030g (8.434mmol) of benzoic acid, 189mg (0.842mmol) of palladium acetate and 4.412g (19.332mmol) of dipotassium hydrogen phosphate in 28mL (0.400mol) of dibromomethane, and stir and react at 130°C for 36 hours. Stirring was stopped, cooled to room temperature, filtered, concentrated under reduced pressure, purified by column chromatography (V PE :V EA =2:1), a white solid—984 mg of the intermediate of formula I was obtained, with a yield of 87%.
[0038]
[0039] The obtained product is analyzed by hydrogen spectrum and carbon spectrum with AVANCF 400MHz nuclear magnetic resonance instrument of German Bruker company, and the characterization results are: 1 H NMR (300MHz, CDCl 3 )δ (ppm): 7.94 (d, J = 7.5Hz, 1H), 7.69 (t, J = 7.5Hz, 1H), 7.49-7.57 (m, 2H), 5.33 (s, 2H); 13 C NMR (100MHz, CDCl 3 )δ (ppm): 171.04, 146.48, 133.96, 128.97, 125.68, 122.06, 69.60....
Embodiment 2
[0057] Taking the synthesis of (±)-4-OH-GR24 as an example, the synthesis method is as follows:
[0058] Steps 1-4 of this embodiment are the same as those of Embodiment 1. In step 5, under an argon atmosphere, 85.6 mg (0.450 mmol) of the intermediate of formula IV was dissolved in 5 mL of dichloromethane, 45.3 mg (0.090 mmol) of iron triflate was added, and the reaction was stirred at room temperature 12 hour, stop stirring, add 20mL dichloromethane for dilution, wash twice with saturated aqueous sodium bicarbonate solution, and wash once with saturated aqueous sodium chloride solution, dry through anhydrous magnesium sulfate, filter, concentrate, and purify by column chromatography (V PE :V EA=1:2) to obtain 54.8 mg of a colorless viscous intermediate of formula V-2 with a yield of 64%.
[0059]
[0060] The obtained product is analyzed by hydrogen spectrum and carbon spectrum with AVANCF 600MHz nuclear magnetic resonance instrument of German Bruker company, and the cha...
Embodiment 3
[0067] Taking the synthesis of (±)-4-SPh-GR24 as an example, the synthesis method is as follows:
[0068] Steps 1-4 of this embodiment are the same as those of Embodiment 1. In step 5, under an argon atmosphere, 83 mg (0.436 mmol) of the intermediate of formula IV was dissolved in 9 mL of dichloromethane, 110 mg (0.218 mmol) of iron triflate, 132 μL (1.308 mmol) of thiophenol were added Stir the reaction at room temperature for 24 hours, stop the reaction, add 20 mL of dichloromethane to dilute, wash twice with saturated aqueous sodium bicarbonate solution, once with saturated aqueous sodium chloride solution, dry over anhydrous magnesium sulfate, filter, concentrate, and column chromatography Purification (V PE :V EA =3:1), to obtain light yellow liquid - 86.2 mg of intermediate of formula V-3, the yield was 70%.
[0069]
[0070] The obtained product is analyzed by hydrogen spectrum and carbon spectrum with AVANCF 400MHz nuclear magnetic resonance instrument of German ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com