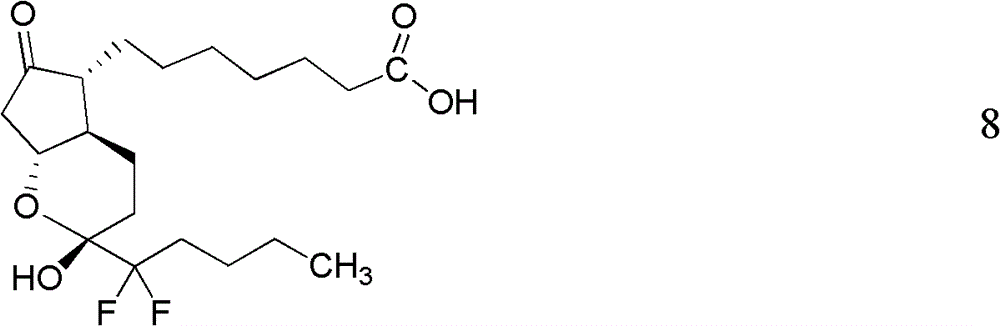
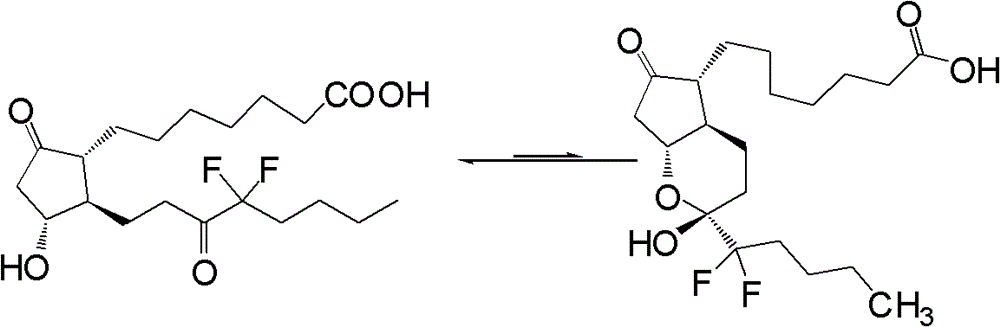
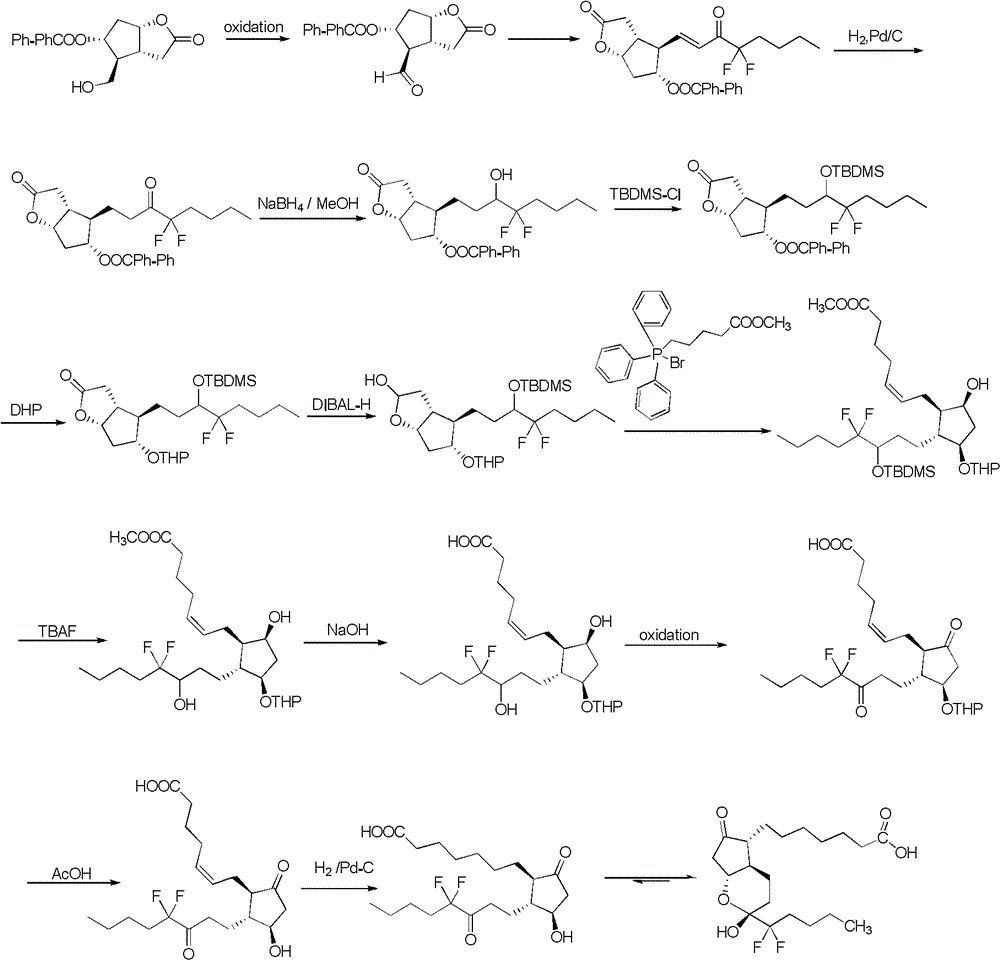
Preparation method of lubiprostone or midbody thereof
A lubiprostone and intermediate technology, which is applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of long steps, high cost, expensive upper side chains, etc., and achieves high reproducibility, improved yield, and easy removal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]
[0065]Compound 1 (50g, 0.29mol) was dissolved in DMF (200mL), cooled to 0°C, imidazole (47.5g, 0.70mol) was added, and then a solution of TBSCl (52.3g, 0.35mol) in DMF (200mL) was slowly added to maintain The temperature of the system does not exceed 10°C. After the addition is complete, stir at 0-5°C for 2h, then add saturated ammonium chloride (200mL) to quench the reaction, extract with dichloromethane until TLC shows that the aqueous layer has no compound 2, add citric acid (10 %, 200mL) and washed twice, the organic phase was separated, washed once with saturated NaCl solution (200mL), washed with saturated sodium bicarbonate solution (200mL), washed with saturated brine (200mL), dried over anhydrous sodium sulfate, and concentrated by rotary evaporation The product was obtained by chromatography (80 g, 96.3% yield).
Embodiment 2
[0067]
[0068] Compound 2 (82g, 0.286mol) was dissolved in dichloromethane (820mL), stirred and dissolved, and 2,2,2-trichloroiminoacetate-4-methoxybenzyl ester (192g, 80%, 0.544mol ), then add CSA (6.64g, 0.0286mol), after the addition is complete, add saturated sodium bicarbonate solution (200mL) after fully reacting at room temperature for 24h, after fully stirring, extract with dichloromethane until TLC shows that the water layer has no compound 3 The organic layers were combined, washed with saturated brine (400mL), dried over anhydrous sodium sulfate, concentrated by rotary evaporation and column chromatography to obtain product 3 (100g, yield 86%).
Embodiment 3
[0070]
[0071] Compound 3 (55g, 135mmol) was dissolved in anhydrous toluene (330mL), cooled to -70°C under nitrogen protection, and DIBAL-H (203mL, 1M in toluene, 203mmol) was added dropwise for 1.5h. Stir at -70°C for 2 h, TLC shows that the reaction is complete, add methanol dropwise to quench the reaction, remove the cooling bath, add saturated sodium potassium tartrate solution (400 mL) dropwise, warm to room temperature, and stir well. Dichloromethane was extracted until TLC showed that the aqueous layer was free of compound 4. The organic layers were combined, washed with saturated brine (400 mL), dried over anhydrous sodium sulfate, concentrated by rotary evaporation, and directly used for the next reaction without purification.
[0072] CEPPA (140g, 317mmol) was suspended in anhydrous THF (200mL), stirred in an ice bath, and LiHMDS (102g, 610mmol) in THF (200mL) was slowly added dropwise at 0°C to obtain a brick red solution. yellow solution. After stirring at 0°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com