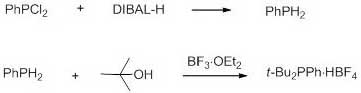
A method for synthesizing di-tert-butylphenylphosphonium tetrafluoroborate
A technology of di-tert-butylphenyl and tetrafluoroboric acid, which is applied in the synthesis of organic phosphine compounds and the synthesis of di-tert-butylphenylphosphonium tetrafluoroborate, which can solve the problems affecting the reaction yield and post-processing difficulties, etc. problem, to achieve the effect of simplifying the experimental steps, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: Synthesis of di-tert-butylphenylphosphonium tetrafluoroborate
[0014] Under the protection of argon, add 1L of toluene to the dry reactor, then add phenylphosphine dichloride (178 g, 1mol), and add 2L of 1.0 M diisobutylaluminum hydride toluene solution dropwise at 0~10 ºC , then heated up to 20-40 ºC for 8 hours, then added 2L of 1N sodium hydroxide solution to quench the reaction, then separated, extracted, and the organic layer was dried with anhydrous magnesium sulfate, and the organic phase was transferred to another dry reaction After the reactor, tert-butanol (148 g, 2 mol) and 47% by mass boron trifluoride ether solution (1.5 kg, 5 mol) were added successively, and after 10 hours of reaction at 20-40 ºC, water was added to quench the reaction. Precipitation was formed, filtered by suction, and the obtained solid was vacuum-dried to obtain 288 g of di-tert-butylphenylphosphonium tetrafluoroborate, with a yield of 93%; 31 P NMR (162 MHz, CDCl 3 , ppm...
Embodiment 2
[0015] Example 2: Synthesis of di-tert-butylphenylphosphonium tetrafluoroborate
[0016] Under the protection of argon, add 1L of toluene to the dry reactor, then add phenylphosphine dichloride (178 g, 1mol), and add 3L of 1.0 M diisobutylaluminum hydride toluene solution dropwise at 0~10 ºC , then heated up to 20-40 ºC for 6 hours, then added 2L of 1N sodium hydroxide solution to quench the reaction, then separated, extracted, the organic layer was dried with anhydrous magnesium sulfate, and the organic phase was transferred to another dry reaction After the reactor, tert-butanol (222 g, 3 mol) and boron trifluoride ether solution (3 kg, 10 mol) with a mass percentage of 47 % were added successively, and after reacting at 20-40 ºC for 10 hours, water was added to quench the reaction. Precipitation was formed, filtered with suction, and the obtained solid was vacuum-dried to obtain 282 g of di-tert-butylphenylphosphonium tetrafluoroborate, with a yield of 91%.
Embodiment 3
[0017] Example 3: Synthesis of di-tert-butylphenylphosphonium tetrafluoroborate
[0018] Under the protection of argon, add 1L of toluene to the dry reactor, then add phenylphosphine dichloride (178 g, 1mol), and add 3L of 1.0 M diisobutylaluminum hydride toluene solution dropwise at 0~10 ºC , then heated up to 20-40 ºC for 8 hours, then added 2L of 1N sodium hydroxide solution to quench the reaction, then separated, extracted, and the organic layer was dried with anhydrous magnesium sulfate, and the organic phase was transferred to another dry reaction After the reactor, tert-butanol (222 g, 3 mol) and 47% by mass boron trifluoride ether solution (1.8 kg, 6 mol) were added successively, and after 12 hours of reaction at 20-40°C, water was added to quench the reaction. Precipitation was formed, filtered with suction, and the obtained solid was vacuum-dried to obtain 288 g of di-tert-butylphenylphosphonium tetrafluoroborate, with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com