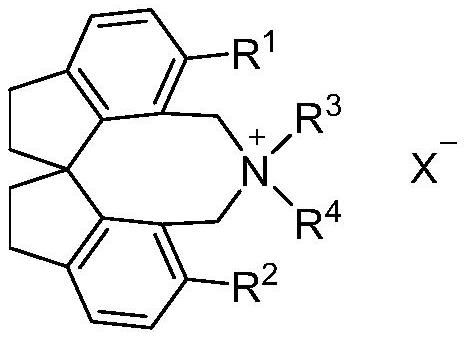
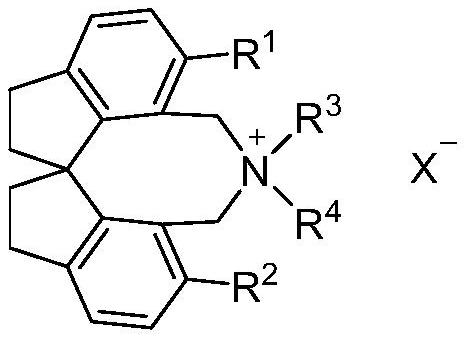
Spiroindane skeleton chiral quaternary ammonium salt as well as preparation method and application thereof
A technology of spiroindane and quaternary ammonium salt, which is applied in the field of chiral quaternary ammonium salt of spirodihydroindane skeleton and its preparation, which can solve the problems of limited range of substrates in asymmetric catalytic reactions and achieve the effect of enriching types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: (R)-12,12-diethyl-1,10-diphenyl-4,5,6,7,12,13-hexahydro-11H-diindeno[7,1-cd : Preparation of 1',7'-ef]azocetine bromide[(R)-Va]
[0030]
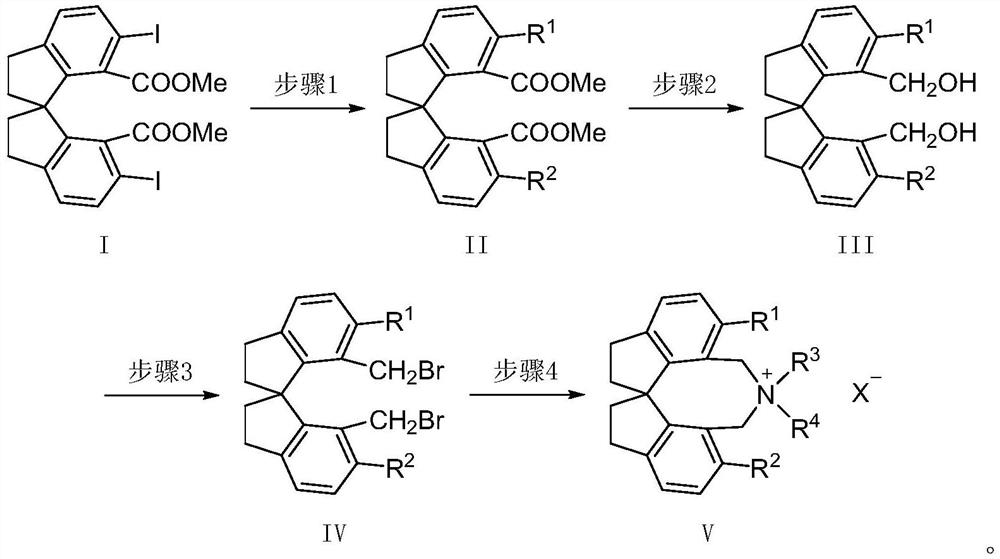
[0031] Step 1, under nitrogen atmosphere, add (R)-6,6'-diiodo-1,1'-spirodihydroindane-7,7'-dicarboxylic acid dimethyl ester [(R)-I, 2mmol] , phenylboronic acid (8mmol), bis(triphenylphosphine)palladium chloride (0.5mmol), potassium carbonate aqueous solution (1.5mol / L, 6mL), methanol (4mL) and tetrahydrofuran (25mL) were sequentially added to a 100mL reaction flask , heated to 85°C and stirred for 24 hours. After the reaction was completed, most of the solvent was evaporated, the residue was diluted with water (20mL), transferred to a separatory funnel, extracted three times with ethyl acetate (50mL), the organic phases were combined, washed once with saturated brine (50mL), added Anhydrous sodium sulfate was allowed to stand for a while. The desiccant was filtered off, most of the solvent was removed by rotary evapo...
Embodiment 2
[0035] Example 2: 1,10-diphenyl-4,5,6,7-tetrahydro-11H,13H-spiro[bisindeno[7,1-cd:1',7'-ef]azocine Preparation of cyclo-12,4'-morpholine] bromide [(R)-Vb]
[0036]
[0037] Step 1, under nitrogen atmosphere, add (R)-6,6'-diiodo-1,1'-spirodihydroindane-7,7'-dicarboxylic acid dimethyl ester [(R)-I, 2mmol] , phenylboronic acid (8mmol), bis(triphenylphosphine)palladium chloride (0.5mmol), potassium carbonate aqueous solution (1.5mol / L, 6mL), methanol (4mL) and tetrahydrofuran (25mL) were sequentially added to a 100mL reaction flask , heated to 85°C and stirred for 24 hours. After the reaction was completed, most of the solvent was evaporated, the residue was diluted with water (20mL), transferred to a separatory funnel, extracted three times with ethyl acetate (50mL), the organic phases were combined, washed once with saturated brine (50mL), added Anhydrous sodium sulfate was allowed to stand for a while. The desiccant was filtered off, most of the solvent was removed by rot...
Embodiment 3
[0041] Example 3: (R)-12,12-diethyl-1,10-bis(4-methylphenyl)-4,5,6,7,12,13-hexahydro-11H-diindeno Preparation of [7,1-cd:1',7'-ef]azococtyl bromide [(R)-Vc]
[0042]
[0043] Step 1, under nitrogen atmosphere, add (R)-6,6'-diiodo-1,1'-spirodihydroindane-7,7'-dicarboxylic acid dimethyl ester [(R)-I, 2mmol] , 4-methylphenylboronic acid (8mmol), bis(triphenylphosphine)palladium chloride (0.5mmol), potassium carbonate aqueous solution (1.5mol / L, 6mL), methanol (4mL) and tetrahydrofuran (25mL) were added to In a 100mL reaction flask, heat up to 90°C and stir for 24 hours. After the reaction was completed, most of the solvent was evaporated, the residue was diluted with water (20mL), transferred to a separatory funnel, extracted three times with ethyl acetate (50mL), the organic phases were combined, washed once with saturated brine (50mL), added Anhydrous sodium sulfate was allowed to stand for a while. The desiccant was filtered off, most of the solvent was removed by rotary...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com