Methods and compositions for inhibition of the transitional endoplasmic reticulum atpase
A technology of compounds and substrates, which can be applied in the direction of drug combination, compound screening, active ingredients of heterocyclic compounds, etc., and can solve problems such as low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0227] Embodiment 1, commercially available compound
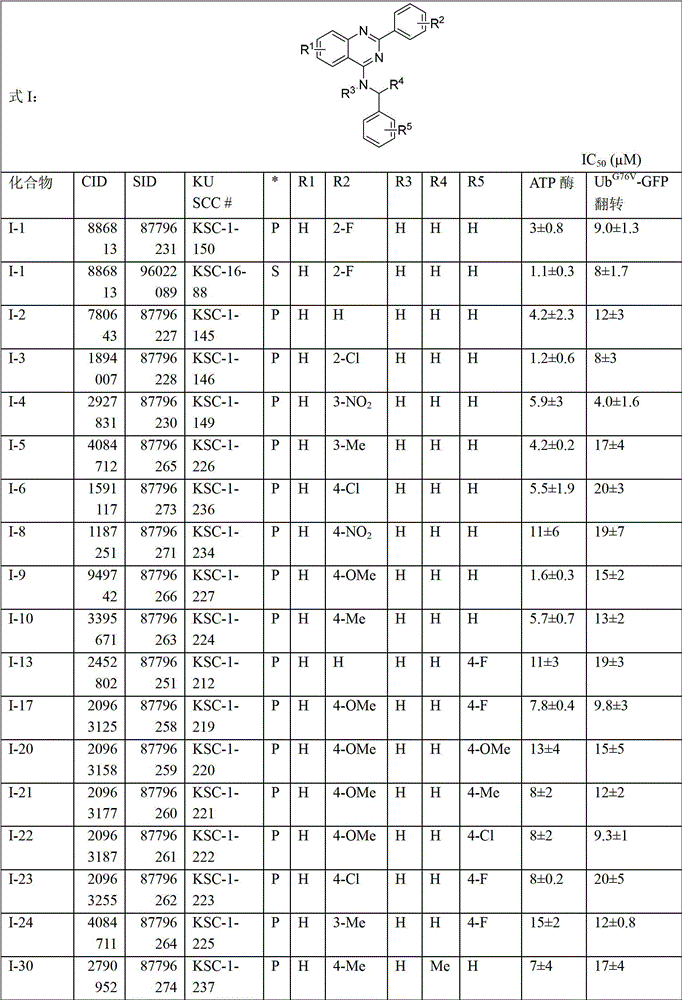
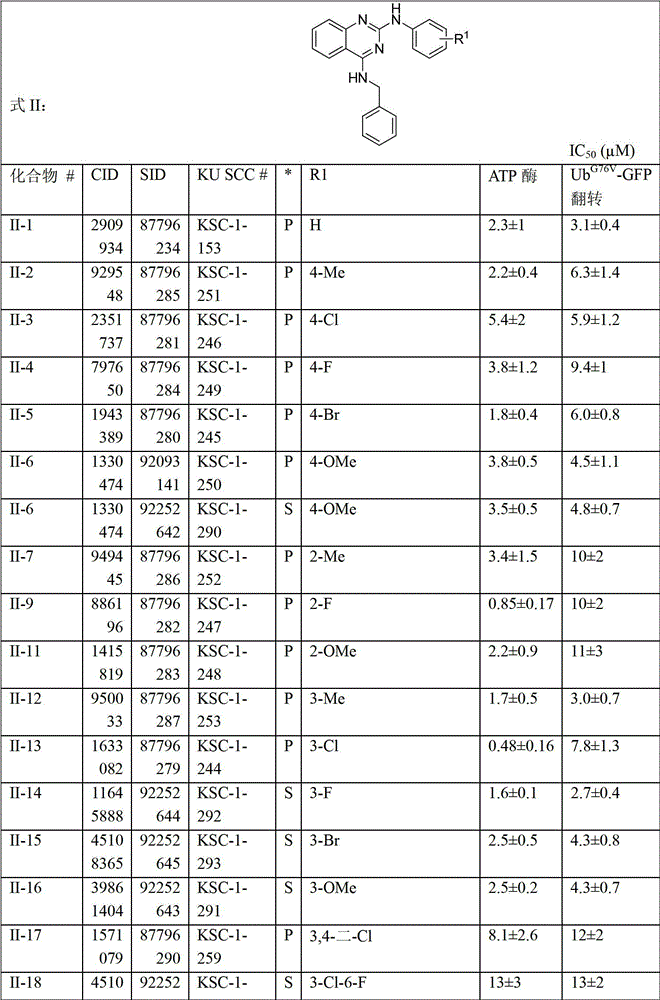
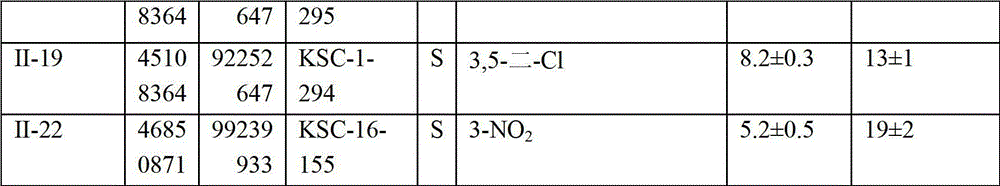
[0228] Compounds shown in Tables 1-33 (as indicated in the table) were either synthesized or commercially available, where S indicates synthetic and P indicates commercially available. Table 34 below provides company information for commercially available compounds.
[0229] Table 34
[0230] compound#
[0231] I-27
Embodiment 2
[0232] Embodiment 2, compound synthesis
[0233] In general, the compounds of the invention (including salts and solvates thereof) can be prepared using known techniques of organic synthesis and can be synthesized according to any of a number of possible synthetic routes. Since the reactions to prepare the compounds of the present invention can be carried out in suitable solvents, the suitable solvents can be easily selected by those skilled in the art of organic synthesis. Suitable solvents can be substantially nonreactive with the starting materials (reactants), intermediates or products at the temperatures at which the reactions are carried out, i.e. temperatures that can vary from the freezing point of the solvent to the boiling point of the solvent . A given reaction can be carried out in one solvent or in a mixture of more than one solvent. Depending on the particular reaction step, a suitable solvent for a particular reaction step can be selected.
[0234] The prepar...
Embodiment 3
[0258] Embodiment 3, the synthesis of compound derivative LII, LIII, LIV, LV and LVI
[0259] Refer to the synthetic scheme of Kohn et al., 1983, J. Am. Chem. Soc, 105, 4106-4108.
[0260]
[0261]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com