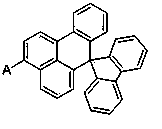
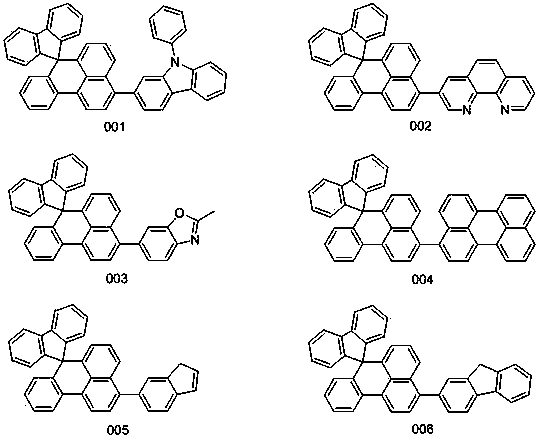
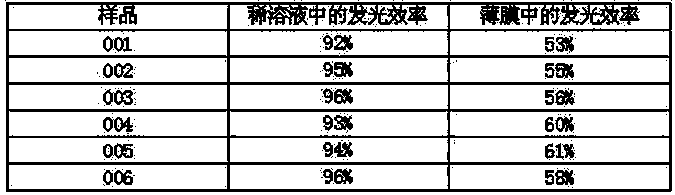
Organic electroluminescent material containing benzanthracene derivative and preparation method of organic electroluminescent material
A technology of benzanthracene and its derivatives, applied in the field of organic electroluminescent materials, to achieve the effect of increasing solubility and easy processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthesis of compound 001
[0022] Concrete synthetic route is as follows:
[0023]
[0024] Add 22.27g (50mmol) of fluorenyl-substituted benzanthracene bromide, 21.69g (75mmol) of N-phenylcarbazolylboronic acid, 10.60g (100mmol) of sodium carbonate, 250ml of tetrahydrofuran and 125ml of water into a three-necked flask and degas , add tetrakis(triphenylphosphine) palladium 0.58g (0.5mmol), raise the temperature to 70°C, react for 15 hours, cool to room temperature, after the solid precipitates, filter with suction, wash the filter cake with water, ethanol and ether, and dry After drying, 27.96 g of asymmetric benzanthracene derivatives were obtained, with a yield of more than 92% and an HPLC purity of more than 98%. Mass Spectrum: Calculated 607.74; Found 607.72. Elemental analysis: Calculated value: C: 92.89%; H: 4.81%; N: 2.30%; Tested value: C: 92.88%; H: 4.83%; N: 2.31%.
Embodiment 2
[0025] Embodiment 2: the synthesis of compound 002
[0026] Concrete synthetic route is as follows:
[0027]
[0028] Add 22.27g (50mmol) of fluorenyl-substituted benzanthracene bromide, 17.92g (80mmol) of phenanthrene boronic acid, 12.19g (115mmol) of sodium carbonate, 250ml of tetrahydrofuran and 125ml of water into a three-necked flask, degas, and add four (three Phenylphosphorus) palladium 0.69g (0.6mmol), heated up to 80°C, reacted for 17 hours, cooled to room temperature, filtered with suction, washed the filter cake with water, ethanol and ether, and dried to obtain asymmetric 25.33 g of benzanthracene derivatives, the yield is over 93%, and the HPLC purity is over 98%. Mass spectrum: calculated value 544.64; found value 544.62. Elemental analysis: calculated value C: 90.42%; H: 4.44%; N: 5.14%; tested value C: 90.41%; H: 4.46%; N: 5.15%.
Embodiment 3
[0029] Embodiment 3: the synthesis of compound 003
[0030] Concrete synthetic route is as follows:
[0031]
[0032] Add 22.27 g (50 mmol) of fluorenyl-substituted benzanthracene bromide, 15.04 g (85 mmol) of 2-methylbenzoxazolylboronic acid, 13.78 g (130 mmol) of sodium carbonate, 250 ml of tetrahydrofuran and 125 ml of water into a three-necked flask, Degas, add tetrakis(triphenylphosphine)palladium 0.81g (0.7mmol), heat up to 85°C, react for 19 hours, cool to room temperature, after the solid precipitates, filter with suction, wash the filter cake with water, ethanol and ether , dried to obtain 23.14 g of asymmetric benzanthracene derivatives, the yield was over 93%, and the HPLC purity was greater than 98%. Mass spectrum: calculated value 497.58; found value 497.56. Elemental analysis: calculated value C: 89.31%; H: 4.66%; N: 2.81%; O: 3.22%; tested value C: 89.32%; H: 4.64%; N: 2.83%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com