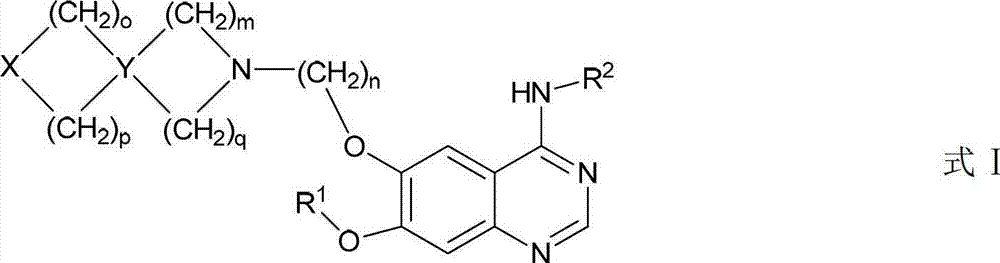
Heterocyclic amino and alkoxy-replaced quinazoline derivative and application thereof
A kind of heterocyclic aminoalkoxy, quinazoline technology, applied in the field of substituted quinazoline derivatives, can solve problems such as difficulty in drug selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0252] The preparation method provided in the present invention should be understood as an example for the sake of full disclosure, rather than limiting the scope of protection required by the present invention. Those skilled in the art can prepare various compounds provided by the present invention according to the guidance of textbooks, experimental manuals or the examples listed in the present invention. The preparation of these compounds also belongs to the general skills that a person of ordinary skill in the art should have, and can also be completed under the guidance of the prior art. Specifically, the preparation method of some compounds provided by the invention is as follows:
[0253] Synthetic method one:
[0254]
[0255] Synthetic method two:
[0256]
Embodiment 1
[0265] Example 1 Synthesis of 4-(3'-chloro-4'-fluoroanilino)-6-[3-(2-oxa-6-azaspiro[3.3]heptane-6-yl) according to synthetic method one -propoxy]-7-methoxyquinazoline (3-C-B)
[0266]
[0267] 1 H NMR (CDCl 3 )8.67(s,1H), 8.03(s,2H), 7.68(br,1H), 7.46(s,1H), 7.25(s,1H), 7.18(m,1H), 4.79(s,4H), 4.25(m,2H), 4.01(s,3H), 3.66(s,4H), 2.80(m,2H), 2.03(m,2H). ES-MS(m / z):459.1(MH + ).
Embodiment 2
[0268] Example 2 Synthesis of 4-(3'-chloro-4'-fluoroanilino)-6-[3-(2-oxa-6-azaspiro[3.4]octane-6-yl according to synthetic method one )-propoxy]-7-methoxyquinazoline (3-C-C)
[0269]
[0270] 1 H NMR (CDCl 3 )8.67(s,1H), 8.20(s,1H), 8.07(q,J=2.4Hz,1H), 7.70(m,1H), 7.57(s,1H), 7.25(s,1H), 7.17( t,J=8.8Hz,1H), 4.69(q,J=6.4Hz,4H), 4.30(t,J=6.8Hz,2H), 4.01(s,3H), 3.18(s,2H), 2.90( t,J=6.4Hz,4H), 2.33(t,J=7.2Hz,2H), 2.22(m,2H). ES-MS(m / z):473.0(MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com