A kind of method of electron-rich five-membered heterocyclic acid and its derivative decarboxylation and fluorine
A five-membered heterocyclic, electron-rich technology, applied in organic chemistry and other fields, can solve problems in the field of organic reactions, such as to be completed, and achieve the effects of optimization of reaction conditions and parameters, high product yield, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
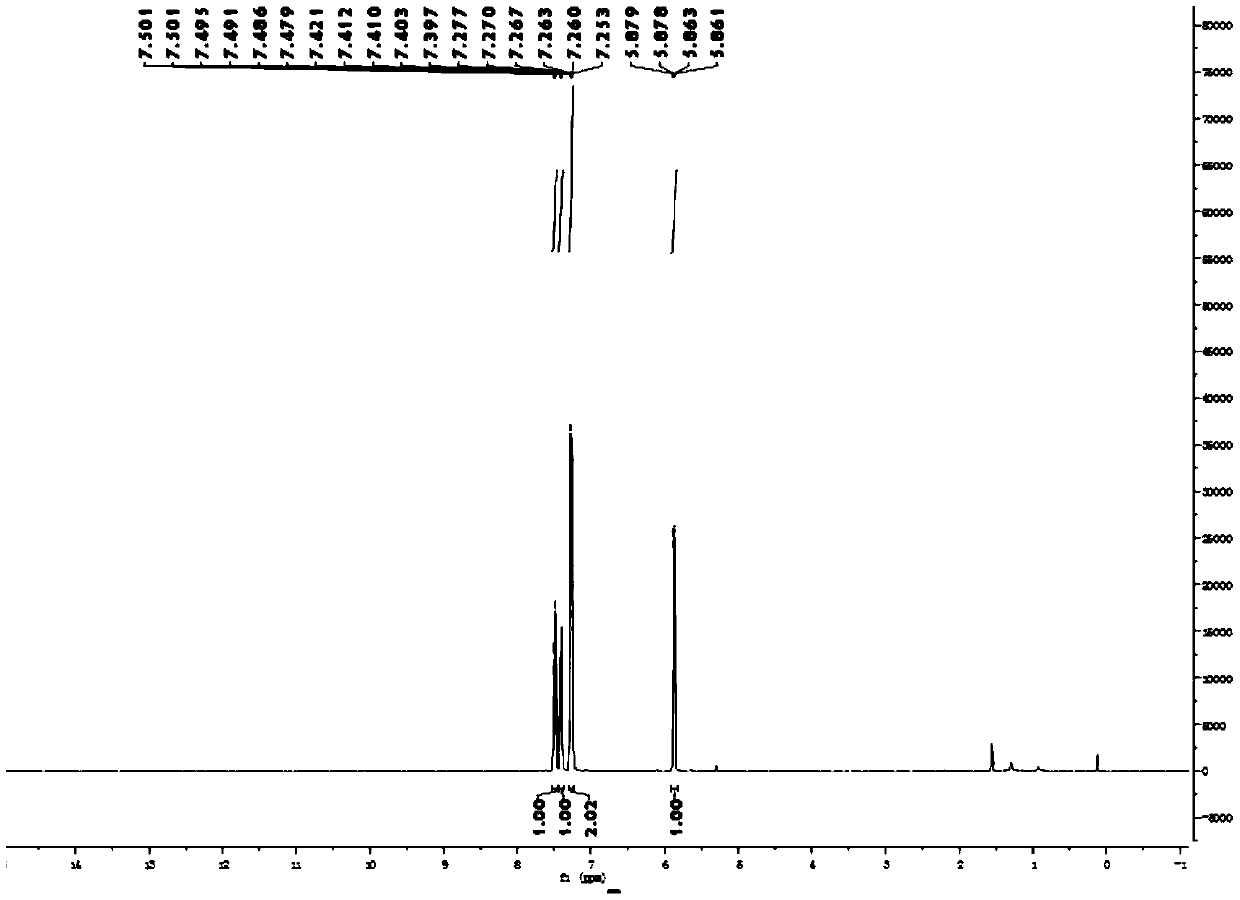
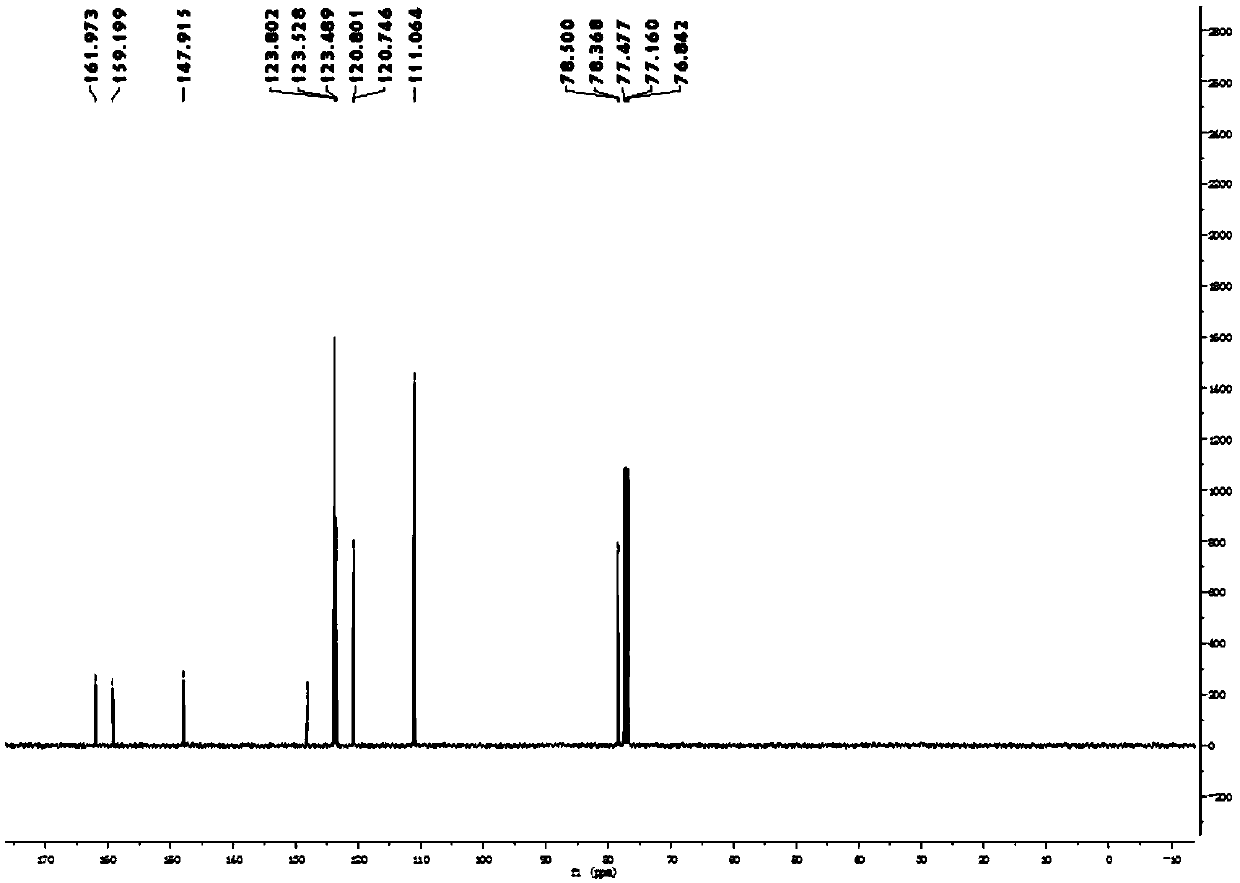
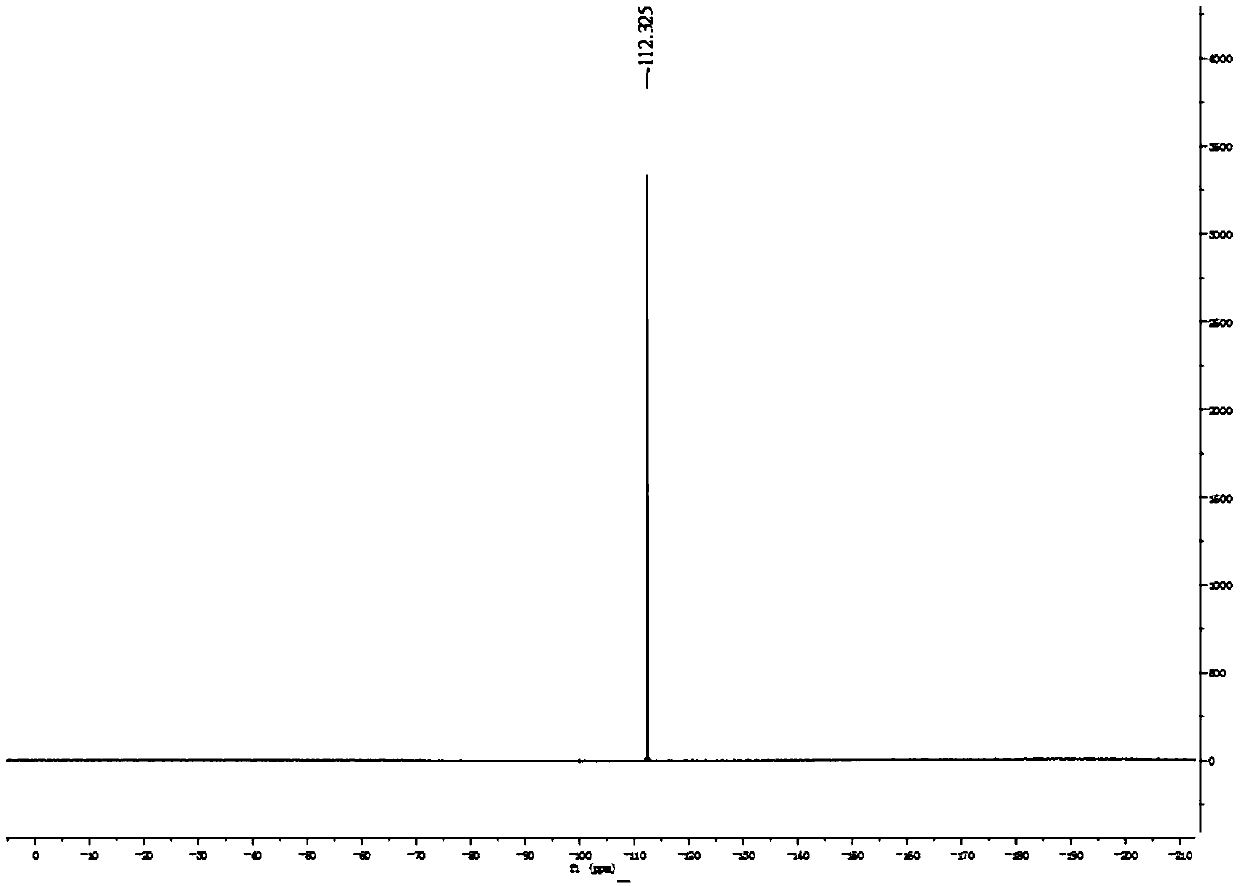
[0055] Synthesis, separation and purification of 2-fluorobenzofuran: Add benzofuran-2-carboxylic acid (162mg, 1.0mmol, 1.0eq) and (708mg, 2.0mmol, 2.0eq), potassium fluoride (232mg, 4.0mmol, 4.0eq); then add dichloroethane (3.3mL) and water (1.7mL) as a solvent; place the reaction bottle at 70°C Heat and stir the reaction in an oil bath pot for 15 hours; after the reaction is completed, extract the reaction mixture system twice with 20 mL ether, combine the organic phases and wash with saturated brine, and then add anhydrous sodium sulfate to dry; after drying, spin dry under reduced pressure organic solvent to obtain a crude product; the crude product was subjected to column separation using analytically pure n-pentane as the eluent to obtain the final product: 2-fluorobenzofuran was a yellow oily liquid with a yield of 75%.
[0056] 1 H NMR (400MHz, CDCl 3 ):δ7.50-7.48(m,1H),7.42-7.40(m,1H),7.28-7.25(m,2H),5.87(dd,J=0.7Hz,6.5Hz,1H). 13 C NMR (100MHz, CDCl 3 ): δ160.6(d,...
Embodiment 2
[0060] Synthesis, separation and purification of 2-fluoro-3-methylbenzofuran: 3-methylbenzofuran-2-carboxylic acid (176mg, 1.0mmol, 1.0 eq), (708mg, 2.0mmol, 2.0eq), potassium fluoride (232mg, 4.0mmol, 4.0eq); then add dichloroethane (3.3mL) and water (1.7mL) as a solvent; place the reaction bottle at 70°C Heat and stir the reaction in an oil bath pot for 15 hours; after the reaction is completed, extract the reaction mixture system twice with 20 mL ether, combine the organic phases and wash with saturated brine, and then add anhydrous sodium sulfate to dry; after drying, spin dry under reduced pressure organic solvent to obtain the crude product; the crude product was separated by column using analytically pure n-pentane as the eluent to obtain the final product: 2-fluoro-3-methylbenzofuran was a light yellow oily liquid with a yield of 63 %.
[0061] 1 H NMR (400MHz, CDCl 3 ): δ7.42-7.40(m,1H),7.36-7.34(m,1H),7.26-7.23(m,2H),2.12(d,J=1.6Hz,3H). 13 C NMR (100MHz, CDCl ...
Embodiment 3
[0065] Synthesis, separation and purification of 5-bromo-2-fluorobenzofuran: 5-bromobenzofuran-2-carboxylic acid (240mg, 1.0mmol, 1.0eq) was successively added into a 50mL round bottom flask equipped with a magnetic stirrer , (708mg, 2.0mmol, 2.0eq), potassium fluoride (232mg, 4.0mmol, 4.0eq); then add dichloroethane (3.3mL) and water (1.7mL) as a solvent; place the reaction bottle at 70°C Heat and stir the reaction in an oil bath pot for 15 hours; after the reaction is completed, extract the reaction mixture system twice with 20 mL ether, combine the organic phases and wash with saturated brine, and then add anhydrous sodium sulfate to dry; after drying, spin dry under reduced pressure Organic solvent to obtain the crude product; the crude product was separated by column using analytically pure n-pentane as the eluent to obtain the final product: 5-bromo-2-fluorobenzofuran was light yellow oily liquid with a yield of 62% .
[0066] 1 H NMR (400MHz, CDCl 3 ): δ7.59(d, J=2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com