Cystamine derivative based on dextran modification as well as preparation and application of cystamine derivative
A technology of dextran and cysteinamide, applied in the field of cysteinamide derivatives, can solve the problems of low drug encapsulation efficiency, high CMC value, and low safety, and achieve high surface activity, reduced adsorption area, solubility and The effect of hardness improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
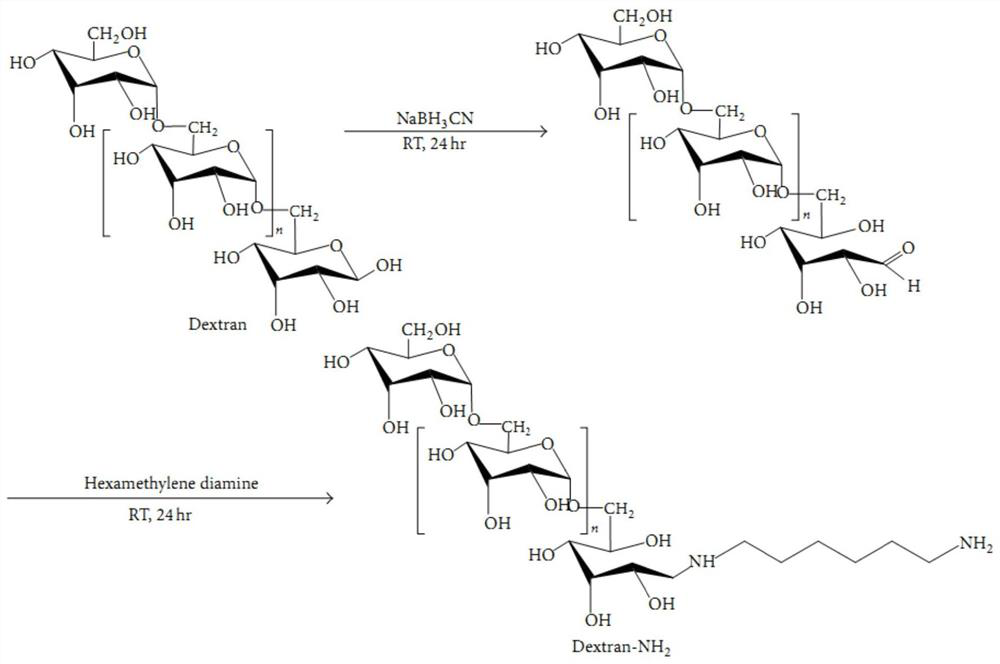
[0041] Dex-NH 2Synthesis: Weigh 4.0g dextran-10 and dissolve it in 50mL dimethyl sulfoxide, add 0.25g sodium cyanoborohydride and 0.20g HDMA to the solution, and stir at room temperature for 24h. Then 0.47 g of hexamethylenediamine was added to the above solution, and the reaction was stirred at room temperature for 24 h. The solution was dialyzed against deionized water for 3 days with a dialysis membrane, concentrated by vacuum rotation and freeze-dried to obtain Dex 10 -NH 2 powder, the synthetic route is as figure 1 shown.
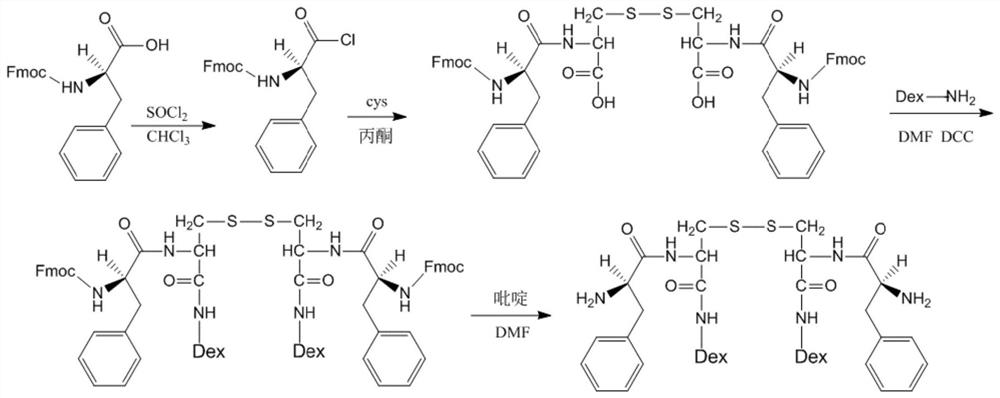
[0042] Synthesis of phenylalanine-cystine-Dex: Weigh 19.38g Fmoc-D-phenylalanine, 20mL chloroform, and 6.0mL thionyl chloride into a 100mL flask, react at 60°C for 2.0h, and depressurize Chloroform and thionyl chloride were distilled off, and then recrystallized from petroleum ether to obtain white crystals of Fmoc-D-phenylalanine acid chloride. Weigh 20.23g of Fmoc-D-phenylalanine acid chloride and dissolve it in 25mL of acetone for later use, ad...
Embodiment 2
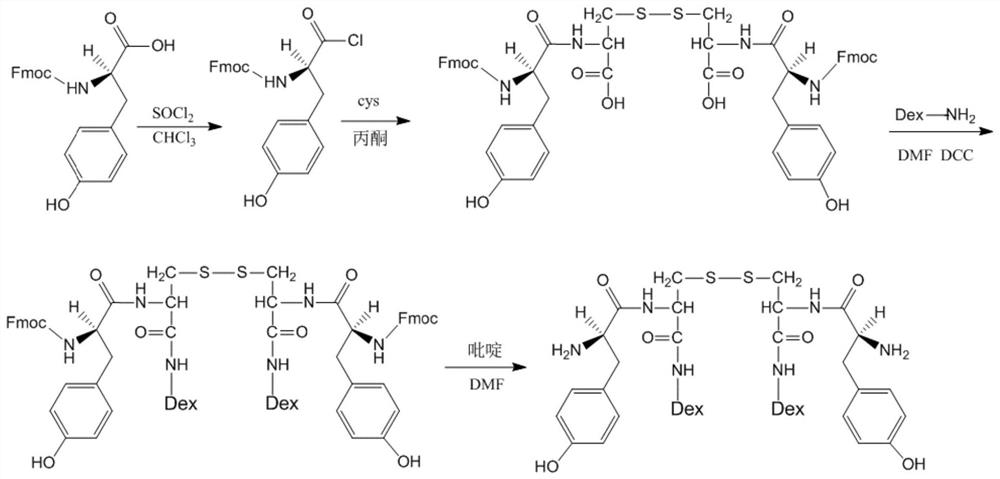
[0044] Synthesis of Tyrosine-Cystine-Dex
[0045] Weigh 12.01g of Fmoc-L-tyrosine, 20mL of chloroform, and 6.0mL of thionyl chloride into a 100mL flask, react at 60°C for 2.0h, evaporate chloroform and thionyl chloride under reduced pressure, and then recrystallize from petroleum ether , to obtain white crystals of Fmoc-L-tyrosine acid chloride. Weigh 21.14g of Fmoc-L-tyrosine acid chloride and dissolve it with 25mL DMF for later use, add 12.01gL-cystine, 20mL concentrated ammonia water and 15mL DMF into a 100mL flask, slowly add Fmoc -L-tyrosine acid chloride DMF solution, warm up to room temperature after the dropwise addition, stir for 4 hours, evaporate DMF under reduced pressure, and recrystallize with ethanol / water / ethyl acetate to obtain Fmoc-L-tyrosine-L-cyst acid. Weigh Fmoc-L-tyrosine-L-cystine 2.4g, Dex 10 -NH 2 (Dextran-10K) 100g was dissolved in 600mL of anhydrous DMF, 1.5g of DCC was added, stirred for 48h, precipitated with excess cold ether, suction filtere...
Embodiment 3
[0047] Synthesis of Nicotinic Acid-Cystine-Dex
[0048] Weigh 5.56g of nicotinic acid, 20mL of chloroform, and 6.0mL of thionyl chloride into a 100mL flask, react at 60°C for 2.0 hours, evaporate chloroform and thionyl chloride under reduced pressure, and then recrystallize petroleum ether to obtain white crystal smoke Acid chloride. Weigh 7.12g of nicotinic acid chloride and dissolve it in 25mL of acetone for later use, add 12.01g of L-cystine, 25.0mL of sodium hydroxide solution (2.5mol / L) and 10mL of acetone in a 100mL flask, and place under ice bath and magnetic stirring conditions , slowly add nicotinic acid chloride acetone solution and 5.0mL sodium hydroxide solution (0.5mol / L) dropwise, warm up to room temperature after the dropwise addition, stir for 4 hours, evaporate acetone under reduced pressure, add dilute sulfuric acid solution dropwise to adjust the solution to Weakly acidic, with solid precipitation, suction filtration, washing, drying, to obtain niacin-L-cys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com