An immunoliposome biochip, its preparation method and its application in biological detection
A technology of immunoliposome and plastid biology, which is applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of inappropriate high-throughput analysis and time-consuming, and achieve the effect of reducing dosage and improving binding ability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
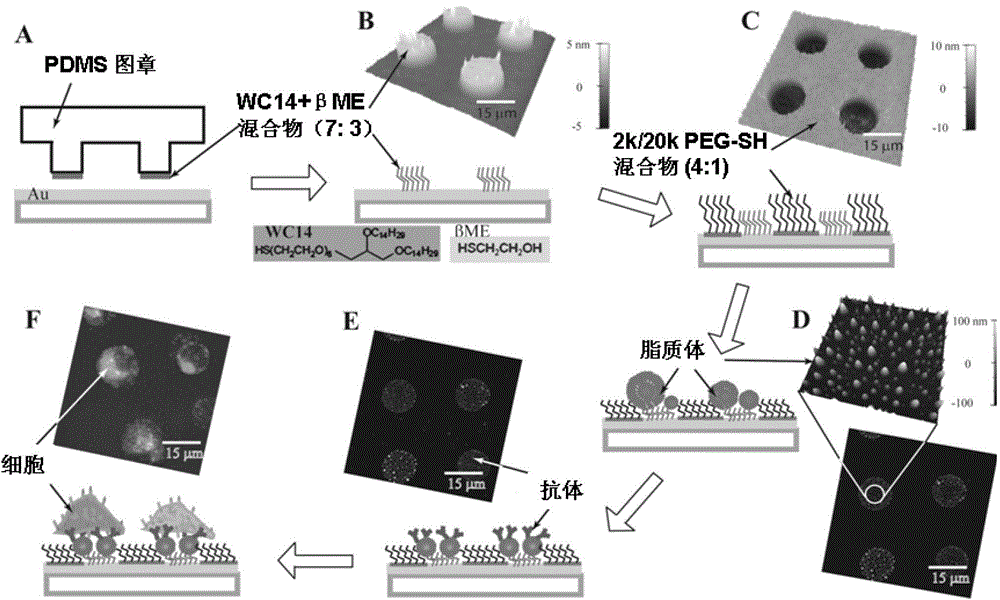
[0038] Example 1: Fabrication of immunoliposome (tILN) biochip
[0039] Example 1 describes a simple method for fabrication of anchored immunoliposome biochips. Such as figure 1 As shown, using a PDMS stamp, an ethanol solution containing 7:3 (molar ratio) of WC14 and β-mercaptoethanol (βME) was transferred to a gold-coated glass substrate by microcontact printing, and the PDMS stamp was fabricated using soft etching techniques. In an atomic force microscope (AFM) image, figure 1 B shows a chip with a diameter of 15 μm, the height of the patterned area is about 4 nm.
[0040] To prevent non-specific cell binding, we invented a mixture of PEG-SH with a molecular weight of 2,000 and 20,000 Daltons in a 4:1 molar ratio to protect 80% of the non-microarray area. In the AFM image, figure 1 The height of PEG-SH shown in C is about 10 nm. Such as figure 1 D, 10 mg / ml lipid mixture [egg phosphatidylcholine (EggPC): cholesterol (CHOL): distearoylphosphatidylethanolamine-polyethy...
Embodiment 2
[0042] Example 2: Isolation of Raji cells from Jurkat cells using an anti-CD20-based chip
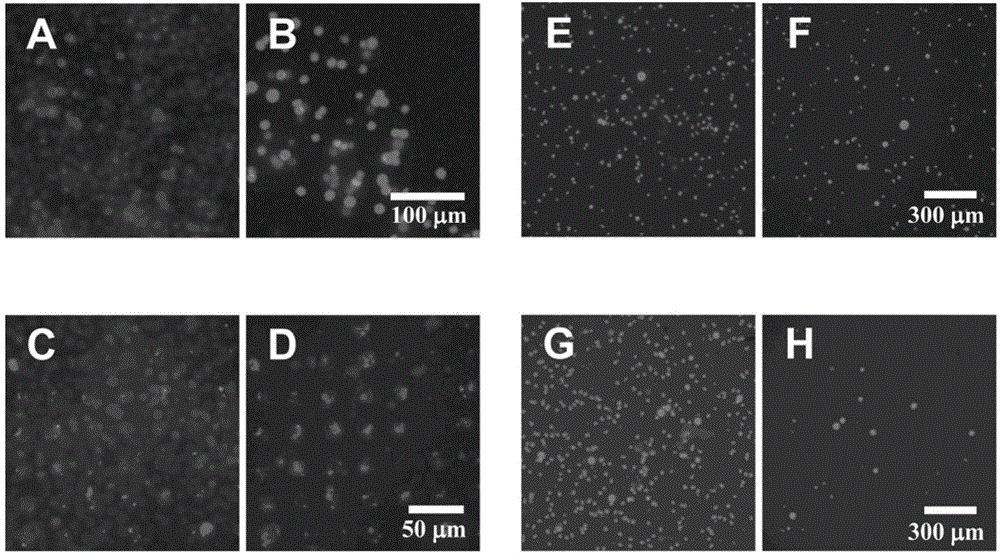
[0043] Example 2 involved the isolation of Raji cells (Burkitt lymphoma B cell line) from Jurkat cells (acute lymphoblastic leukemia cell line) using a cell surface ligand chip containing Rituximab (anti-CD20 monoclonal antibody rituximab). figure 2 Among them, (A) to (D) fluorescence microscope images show the comparison of the results of capturing cells with conventional antibodies and the immunoliposome biochip prepared in Example 1. Apparently, the effect of isolating Raji cells with immunoliposome biochip is much better.
Embodiment 3
[0044] Example 3: Isolation of MCF-7 cells from Raji cells with EpCAM antibody-based chip
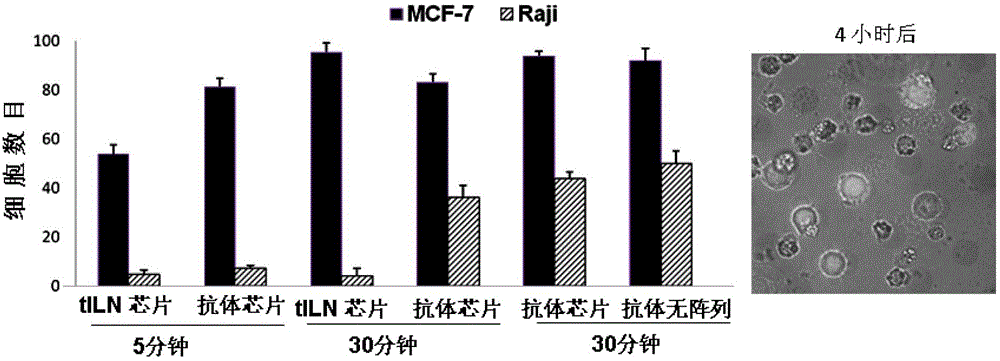
[0045] Example 3 is the application of the immunoliposome biochip prepared in Example 1 to the isolation of rare cells, such as circulating tumor cells (CTCs) in blood samples, which are very valuable for early clinical diagnosis and non-invasive cancer metastasis Prognostic indicators. However, it is still a huge challenge to detect CTCs in medicine, mainly because the number of such cells in the blood of patients is very small. Since CTCs express epithelial cell adhesion molecule (EpCAM) on their surface, but normal blood cells do not, immobilized anti-EpCAM has been used on CTCs isolated from patient blood. In this example, our target simulates blood cells to separate a very small amount of MCF-7 cells from a large number of Raji cells. At the same time, because the overexpression of miR-21 in breast cancer cells can be used as a viable biomarker. Using these cell lines at differe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com