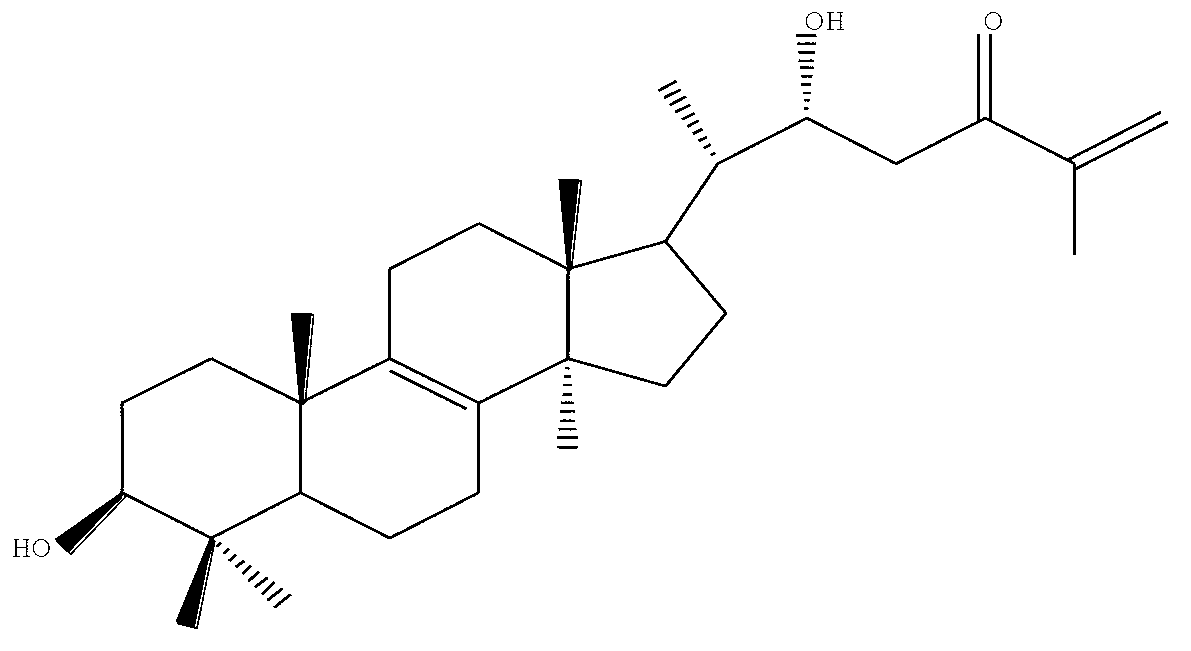
Application of 3beta, 22alpha-dihydroxylanosta-8,25-diene-24-one in preparing antiviral medicament
Technology of an antiviral drug, lanosterol, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Isolation and Purification of 3β,22α-Dihydroxylanoster-8,25-dien-24-one from Fruiting Body of Inonotus obliquus
[0022] 1. Collect 3Kg Inonotus obliquus sporocarp raw material, after drying and crushing, extract with 95% ethanol at room temperature, the ratio of solid to liquid is 1:20, extract 3 times in total for 2 hours each time; collect and extract Extract the liquid and vacuum dry and concentrate to obtain 780g of dark brown ethanol extract extract, add 1000ml of water to dissolve and mix, add 3000ml of ethyl acetate organic solvent, extract 3 times, collect the ethyl acetate extract and concentrate in vacuo to obtain Ethyl acetate extract 111.7g, water soluble matter 650g.
[0023] 2. Take 111.7g of the ethyl acetate extract, and perform normal phase silica gel column chromatography. The ratio of silica gel for sample mixing is 1:1.5, and the ratio of silica gel for upper column is 1:20. The inner diameter of the column is 12.5cm, and the gradient elution is: ch...
Embodiment 2
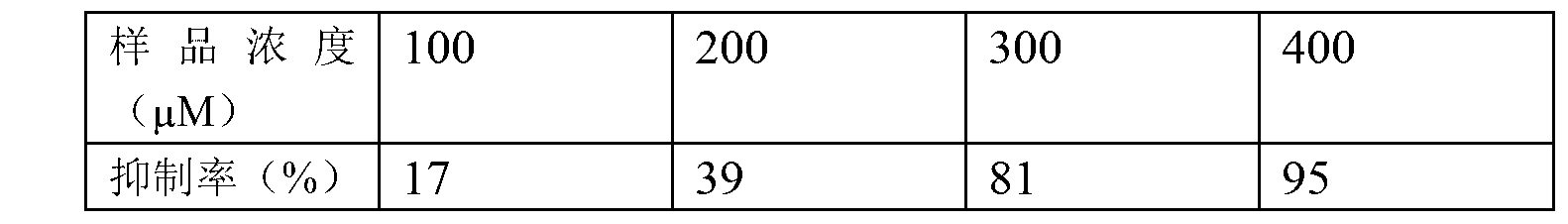
[0028] Inhibitory effect of 3β,22α-dihydroxylanoster-8,25-dien-24-one on simian HIV (SIV)
[0029] Dissolve the 3β,22α-dihydroxylanoster-8,25-dien-24-one obtained in Example 1 with dimethyl sulfoxide (DMSO) to prepare a mother solution, and then dilute it with cell culture medium RPMI-1640 to obtain the For the desired concentration, sample solutions with different concentrations were obtained, and an equal amount of CEM×174 cell and virus mixture was added. Each gradient was repeated 3 times, and after occluded bodies appeared (after 4 days of action), the cell clusters were blown to disperse them. Calculate the number of clathrates per well. Take 6 averages for each such gradient.
[0030]Inhibition rate = (the number of inclusions in the control hole - the number of inclusions in the drug administration hole) / the number of inclusions in the control hole × 100% inhibition rate The effect is shown in Table 1.
[0031] Table 1
[0032]
Embodiment 3
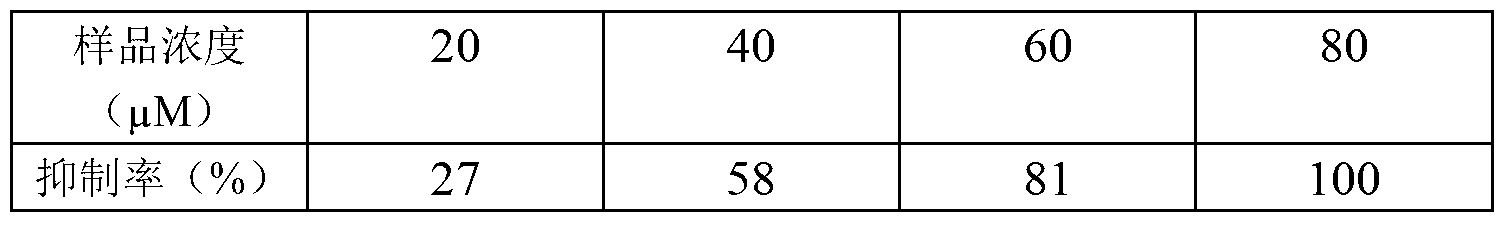
[0034] Inhibitory effect of 3β,22α-dihydroxylanoster-8,25-dien-24-one on herpes simplex virus (HSV)
[0035] Reporter virus assay: Dissolve the 3β,22α-dihydroxylanoster-8,25-dien-24-one obtained in Example 1 with dimethyl sulfoxide (DMSO) to prepare the mother solution, and then use the cell culture medium RPMI- 1640 was diluted to the required concentration to obtain sample solutions of different concentrations, mixed with HSV-Blue reporter virus and sample solutions of different concentrations, added to vero cells, incubated for 24 hours, discarded the mixture of samples and viruses, and added to each well 100ul of 1% NP40 cell lysate, placed for 5-10min, and oscillating three times in the middle. Pipette 50ul of lysate per well and add to 96-well ELISA plate (or 96-well cell culture plate), then add 50ul of β-Gal activity detection substrate buffer, shake gently to mix. The determination of dynamic light absorption at 570nm was carried out on a super microplate reader. Th...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap