Tumor targeting polypeptide-polyethylene glycol-polylactic acid compound, preparation method thereof and applications thereof
A polyethylene glycol and tumor-targeting technology, which is applied in the direction of non-active ingredients of polymer compounds, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of poor delivery effect, low targeting efficiency, and no targeting materials yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
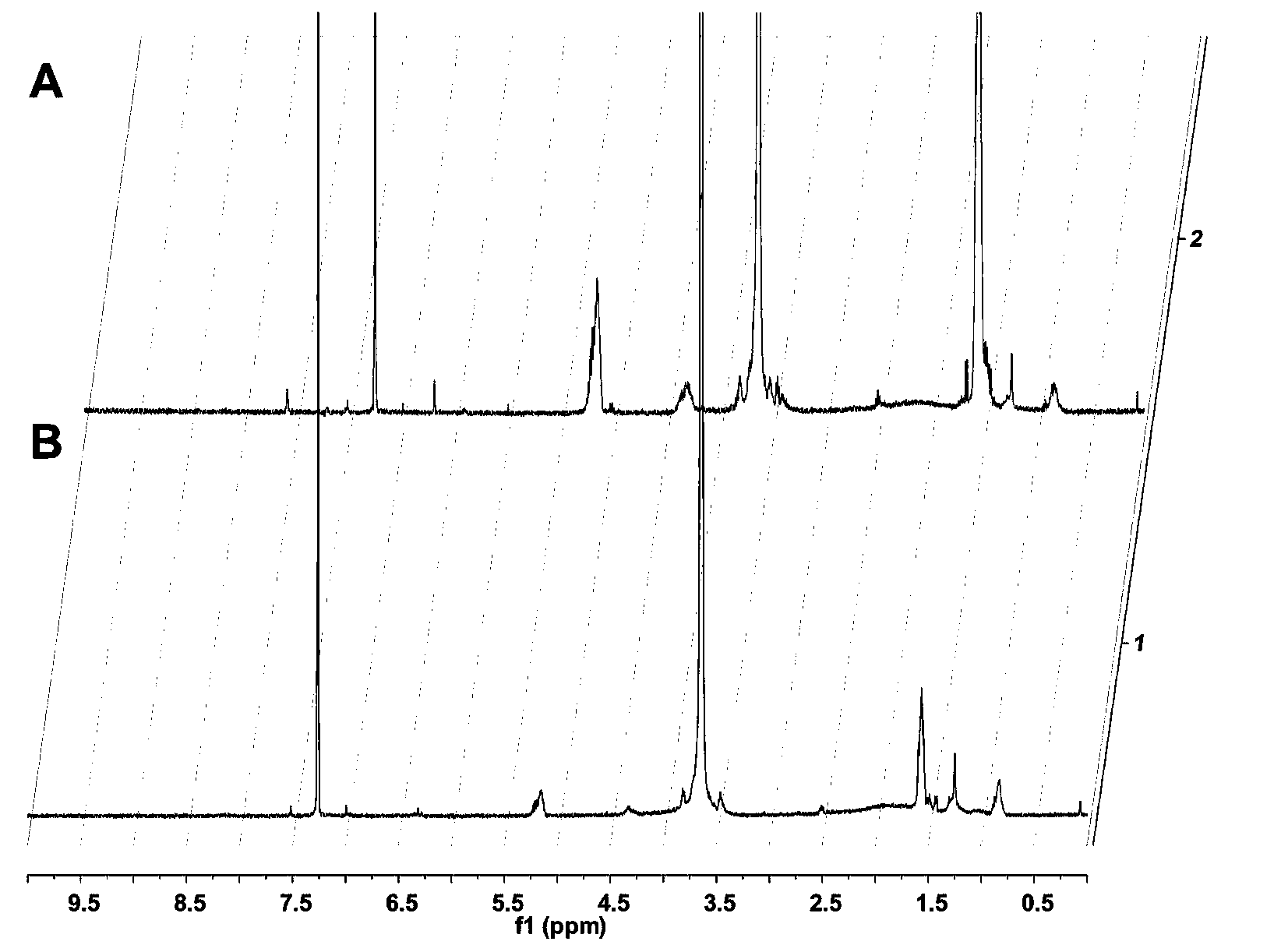
[0019] Example 1: Synthesis, purification and characterization of targeting material APARPAR-PEG-PLA.
[0020] tert-Butoxycarbonyl-arginine (p-tosyl)-p-acetamidobenzyl ester) Boc-Arg(Tos)-PAM resin was deprotected with trifluoroacetic acid (TFA) for 1 min twice, and the Boc-protected amino acid Dissolve in 0.5M HBTU (solvent is DMF), react at room temperature for 15 minutes, wash with DMF, remove Boc protection with TFA, react and connect all amino acids sequentially according to the amino acid sequence. After the reaction, wash the resin, remove the protective group with TFA, and dry it in vacuum. Then put it into a peptide cutting tube, add an appropriate amount of P-cresol, then pass it into HF, stir and react in an ice bath for 1 hour; after the reaction, remove the HF in the tube under reduced pressure. , the residual solution was precipitated with an appropriate amount of glacial ether, filtered to obtain the precipitate and washed with glacial ether; the precipitate was...
Embodiment 2
[0021] Example 2: Synthesis, purification and characterization of targeting material APARPAR-PEG-PLA.
[0022] tert-Butoxycarbonyl-arginine (p-tosyl)-p-acetamidobenzyl ester) Boc-Arg(Tos)-PAM resin was deprotected with trifluoroacetic acid (TFA) for 1 min twice, and the Boc-protected amino acid Dissolve in 0.5M HBTU (solvent is DMF), react at room temperature for 15 minutes, wash with DMF, remove Boc protection with TFA, react and connect all amino acids sequentially according to the amino acid sequence. After the reaction, wash the resin, remove the protective group with TFA, and dry it in vacuum. Then put it into a peptide cutting tube, add an appropriate amount of P-cresol, then pass it into HF, stir and react in an ice bath for 1 hour; after the reaction, remove the HF in the tube under reduced pressure. , the residual solution was precipitated with an appropriate amount of glacial ether, filtered to obtain the precipitate and washed with glacial ether; the precipitate was...
Embodiment 3
[0023] Example 3: Synthesis, purification and characterization of targeting material APARPAR-PEG-PLA.
[0024] tert-Butoxycarbonyl-arginine (p-tosyl)-p-acetamidobenzyl ester) Boc-Arg(Tos)-PAM resin was deprotected with trifluoroacetic acid (TFA) for 1 min twice, and the Boc-protected amino acid Dissolve in 0.5M HBTU (solvent is DMF), react at room temperature for 15 minutes, wash with DMF, remove Boc protection with TFA, react and connect all amino acids sequentially according to the amino acid sequence. After the reaction, wash the resin, remove the protective group with TFA, and dry it in vacuum. Then put it into a peptide cutting tube, add an appropriate amount of P-cresol, then pass it into HF, stir and react in an ice bath for 1 hour; after the reaction, remove the HF in the tube under reduced pressure. , the residual solution was precipitated with an appropriate amount of glacial ether, filtered to obtain the precipitate and washed with glacial ether; the precipitate was...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap