Resolution of 1-(4-methoxybenzyl)-octahydro-isoquinoline
A technology of methoxybenzyl and methoxy, which is applied in the field of resolution of 1--octahydro-isoquinoline, and can solve problems such as similar efforts of octabasic analogues that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
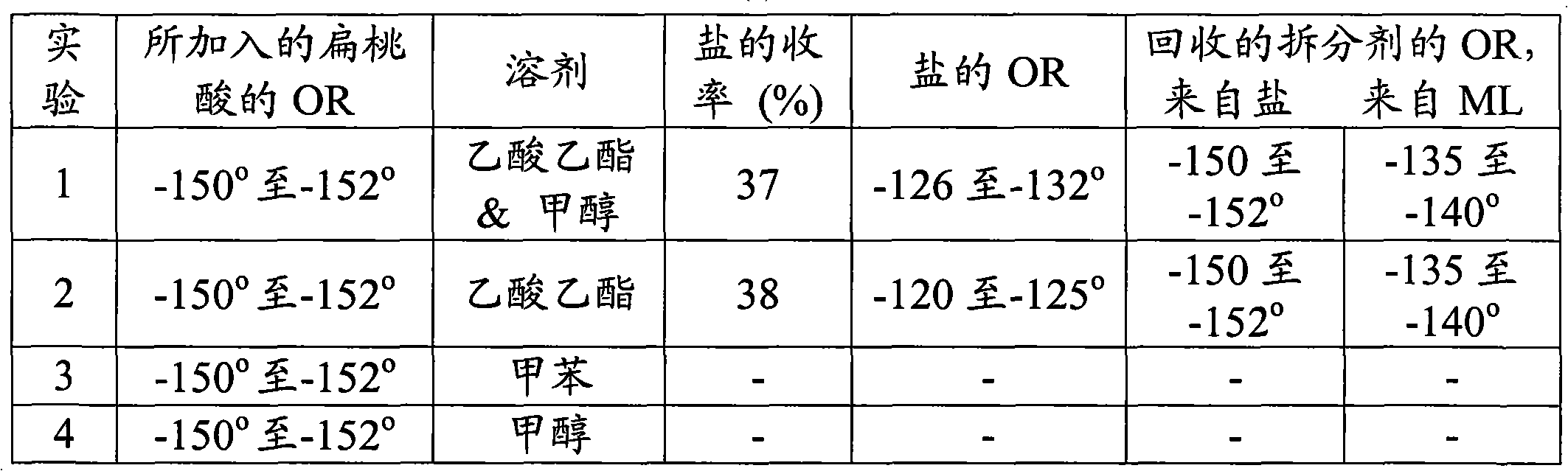
[0030] Example 1: Resolution of (±)-1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydro-isoquinoline:
[0031] Solid R-naproxen (36.4 g, 0.157 moles) was added to a solution of racemic octabasic (50.0 g, 0.1942 moles) in 300 ml of toluene. The reaction mixture was heated to 45-55 °C and then stirred at the same temperature for 1 h. The monolith was allowed to cool to 25-35°C for about 30 minutes, further cooled to 6-14°C and aged at the same temperature for 2h. The (-) octabasic-R-naproxen salt thus formed was filtered at 6-14°C and the wet cake was washed with 30 ml of toluene. It was then purified by slurrying in toluene at 40-50 °C for about 1 h, cooled to 25-35 °C, then further cooled to 6-14 °C, stirred for 2 h, filtered and washed with toluene (4.5 ml) and dried to give crystalline (-) octabasic-R-naproxen salt (32.0 g, 33.8% yield) having an optical rotation of (-) 70 to 75° at 20°C, 589 nm, in 1% methanol. The mother liquor containing the undesired enantiomer (+ octabasic...
Embodiment 2
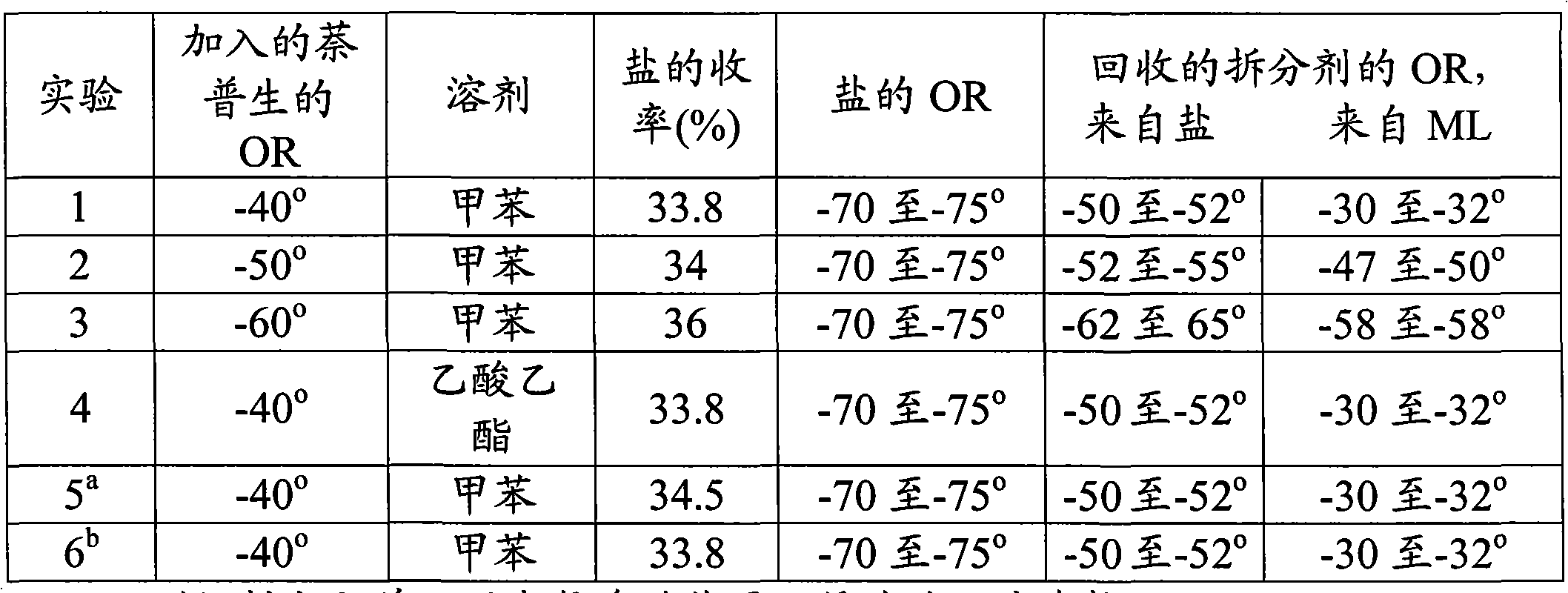
[0032] Example 2: Resolution of (±)-1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydro-isoquinoline:
[0033] The racemic octabasic HBr salt (131.5 g) was added to a flask containing 500 ml of water, cooled to 10-15 °C, the pH was adjusted to 12 to 13 using sodium hydroxide lye and 325 ml of toluene was added to extract The free racemic octabasic compound released. The toluene layer was washed with water and the organic layer was dried. The toluene layer containing 100.0 g (0.388 moles) of free octabasic was charged to a flask and 200 ml of toluene was added. Solid R-naproxen (72.8 g, 0.316 moles, rotation about -40°) was added to the solution. The reaction mixture was heated to 45-55 °C, stirred for 1 h, then cooled to 25-35 °C, maintained for about 30 min, further cooled to 6-14 °C and maintained at the same temperature for 2 h. The (-) octabasic-R-naproxen salt thus formed was filtered at 6-14°C and the wet cake was washed with 60 ml of toluene. The mother liquors were colle...
Embodiment 3
[0035] Example 3: Decomposition of (±)-1-(4-methoxybenzyl)-1,2,3,4,5,6,7,8-octahydro-isoquinoline:
[0036] The toluene layer containing about 100 g (0.388 moles) of the racemic octabasic compound as in Example 2 above was concentrated under vacuum below 50 °C to remove the toluene to give the crude racemic octabasic group. To this was added enough ethyl acetate to bring the total volume to 400ml. To this solution was added solid R-naproxen (72.8 g, 0.316 mol, optical rotation about -40°). The reaction mixture was heated to 45-55 °C, stirred for 1 h, then cooled to 25-35 °C, maintained for about 30 min, further cooled to 6-14 °C and maintained at the same temperature for 2 h. The (-) octabasic-R-naproxen salt thus formed was filtered at 6-14°C and the wet cake was washed with 60 ml of ethyl acetate. The mother liquors were collected separately for recovery of R-naproxen, DL-octabasic and (+)-octabasic.
[0037] The (-) octabasic-naproxen salt was slurried in ethyl acetate a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com