Culture medium suitable for preparing porcine circovirus type II vaccine through PK15 cell and using method thereof
A virus vaccine and culture medium technology, applied in the field of biological products, can solve the problems of difficult control of product batch differences, less research on culture medium, and difficult to clarify components, and achieve the effects of good cell shape, reduced risk, and fast growth rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The medium of the PK15 cell large-scale high-density culture that uses, its composition and the ratio of parts by weight are as follows:
[0053]
[0054]
[0055] 2% fetal bovine serum was added to the above medium to prepare a medium for large-scale high-density culture of PK15 cells.
[0056] The composition of the medium used to maintain virus proliferation and the ratio of parts by weight are as follows:
[0057]
[0058]
[0059] On the basis of the above-mentioned medium, the following components are also added to make a culture solution for maintaining virus proliferation, and the ratio of its parts by weight is as follows:
[0060]
[0061]
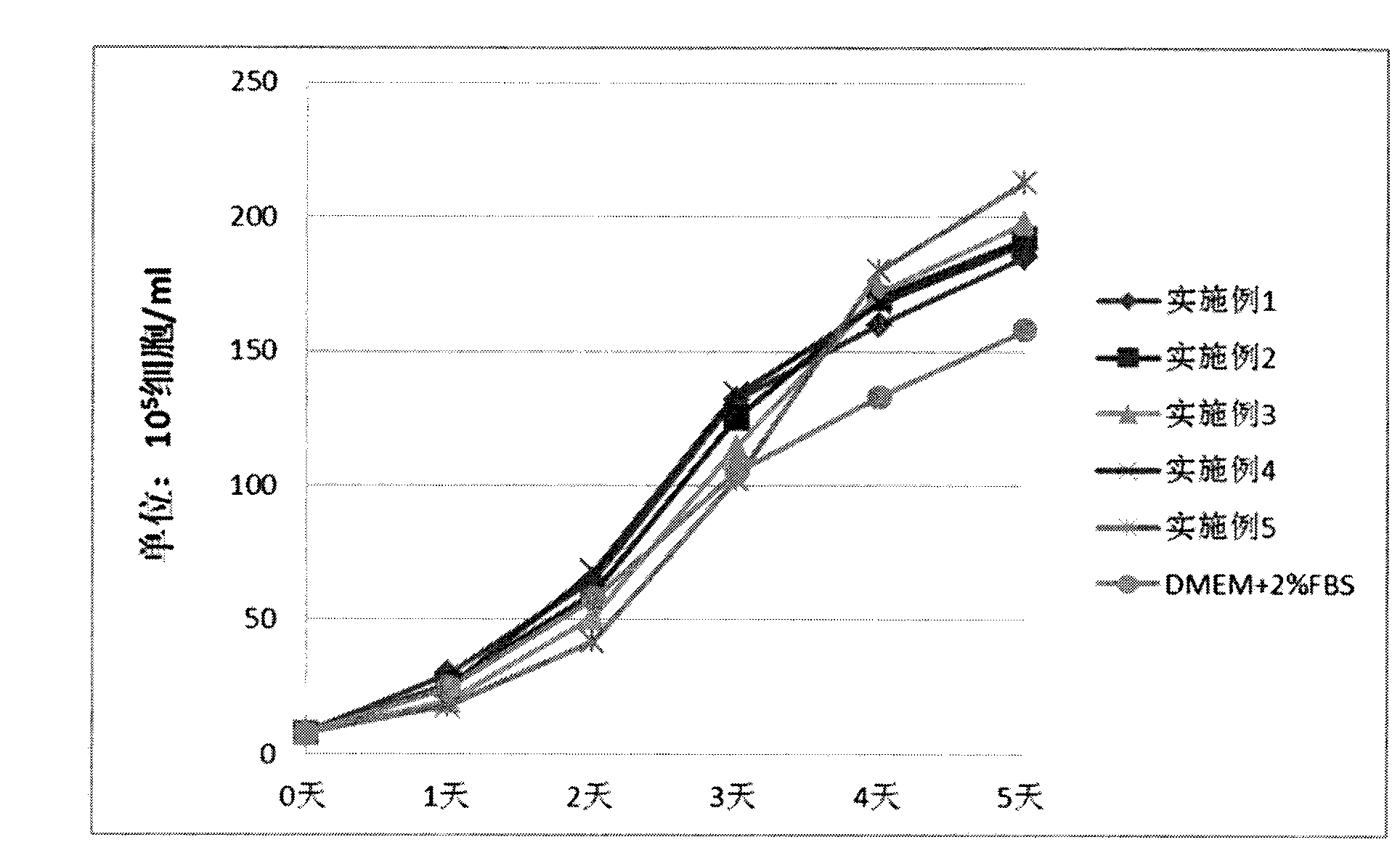
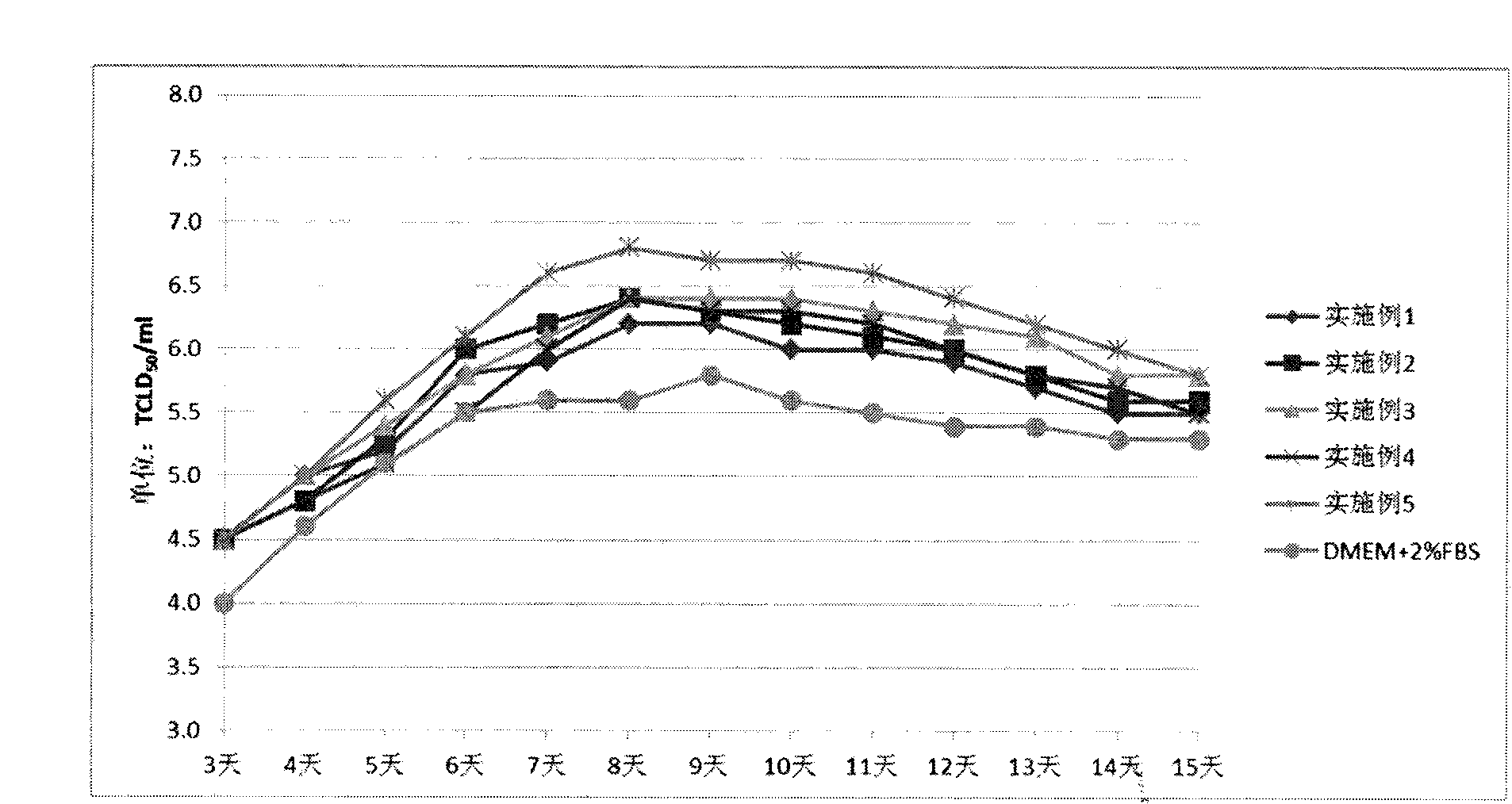
[0062] The experimental results are shown in Table 1, the data of "Example 1" in Table 2.
Embodiment 2
[0064] The medium of the PK15 cell large-scale high-density culture that uses, its composition and the ratio of parts by weight are as follows:
[0065]
[0066]
[0067] 2% fetal bovine serum was added to the above medium to prepare a medium for large-scale high-density culture of PK15 cells.
[0068] The composition of the medium used to maintain virus proliferation and the ratio of parts by weight are as follows:
[0069]
[0070]
[0071] On the basis of the above-mentioned medium, the following components are also added to make a culture solution for maintaining virus proliferation, and the ratio of its parts by weight is as follows:
[0072]
[0073] The experimental results are shown in Table 1 and the data of "Example 2" in Table 2.
Embodiment 3
[0075] The medium of the PK15 cell large-scale high-density culture that uses, its composition and the ratio of parts by weight are as follows:
[0076]
[0077]
[0078] 2% fetal bovine serum was added to the above medium to prepare a medium for large-scale high-density culture of PK15 cells.
[0079] The composition of the medium used to maintain virus proliferation and the ratio of parts by weight are as follows:
[0080]
[0081]
[0082] On the basis of the above-mentioned medium, the following components are also added to make a culture solution for maintaining virus proliferation, and the ratio of its parts by weight is as follows:
[0083]
[0084] The experimental results are shown in Table 1, the data of "Example 3" in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com