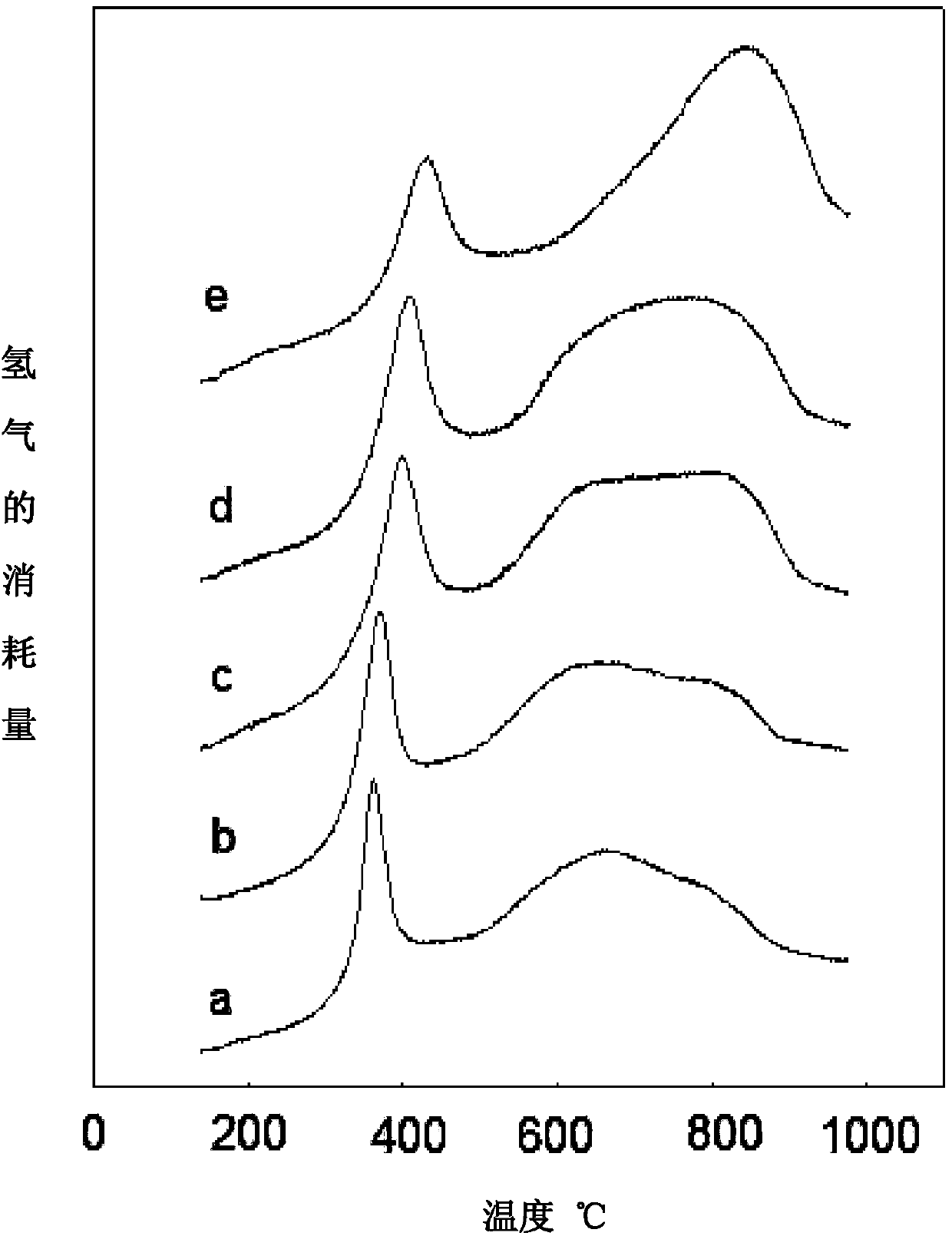
ZrO2-loaded high-stability sulfur-tolerant methanation catalyst
A sulfur methanation-resistant, high-stability technology, applied in physical/chemical process catalysts, catalyst supports, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as failure to reach
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
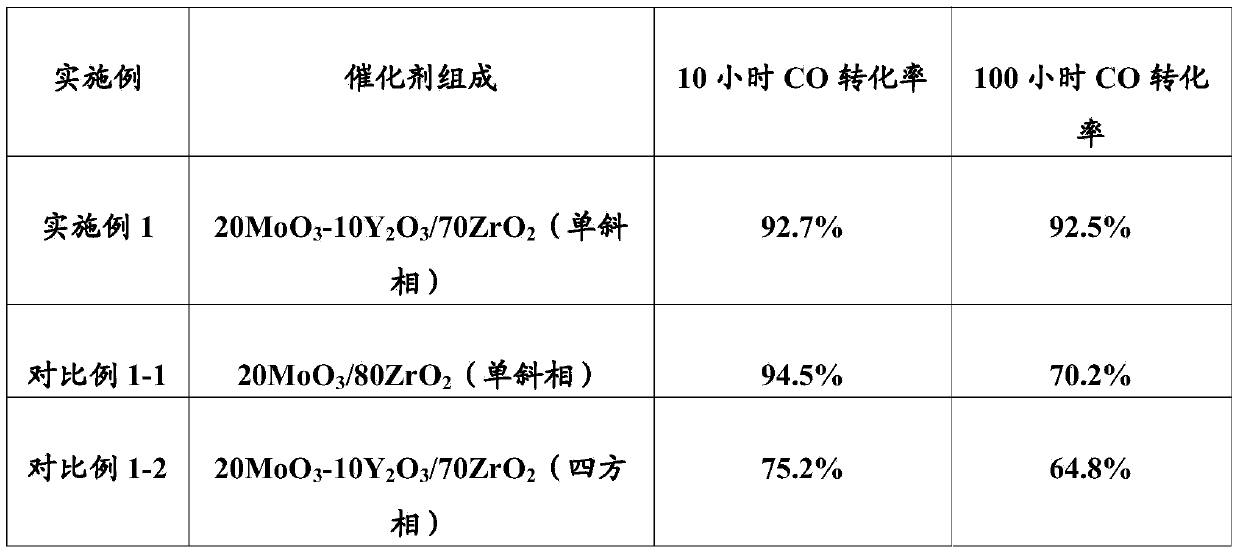
[0073] The following examples have no special instructions, and the ratios or parts of materials are weight ratios or parts. Example 1: Preparation of 20MoO by impregnation method 3 -10Y 2 o 3 / 70ZrO 2 (monoclinic) catalyst
[0074] (1) 8.48 grams of Y (NO 3 ) 3 .6H 2 O was dissolved in 60 g of deionized water and stirred to form an impregnation solution. Weigh 70 g of commercially available monoclinic ZrO 2 Carrier (with a specific surface area of 90m 2 / g), add it to the impregnation solution, stir vigorously for 2 hours to form a uniform suspension, evaporate the water to dryness with a rotary evaporator, and then put it in a 110°C drying oven to dry for 12 hours. Finally, Baked in a muffle furnace at 600 °C for 4 hours to obtain surface-loaded Y 2 o 3 Monoclinic ZrO 2 porous carrier.
[0075] (2) 24.5 grams of ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 4H2 O) be dissolved in 60 grams of deionized water, and be made into dipping solution through stirring. T...
Embodiment 2
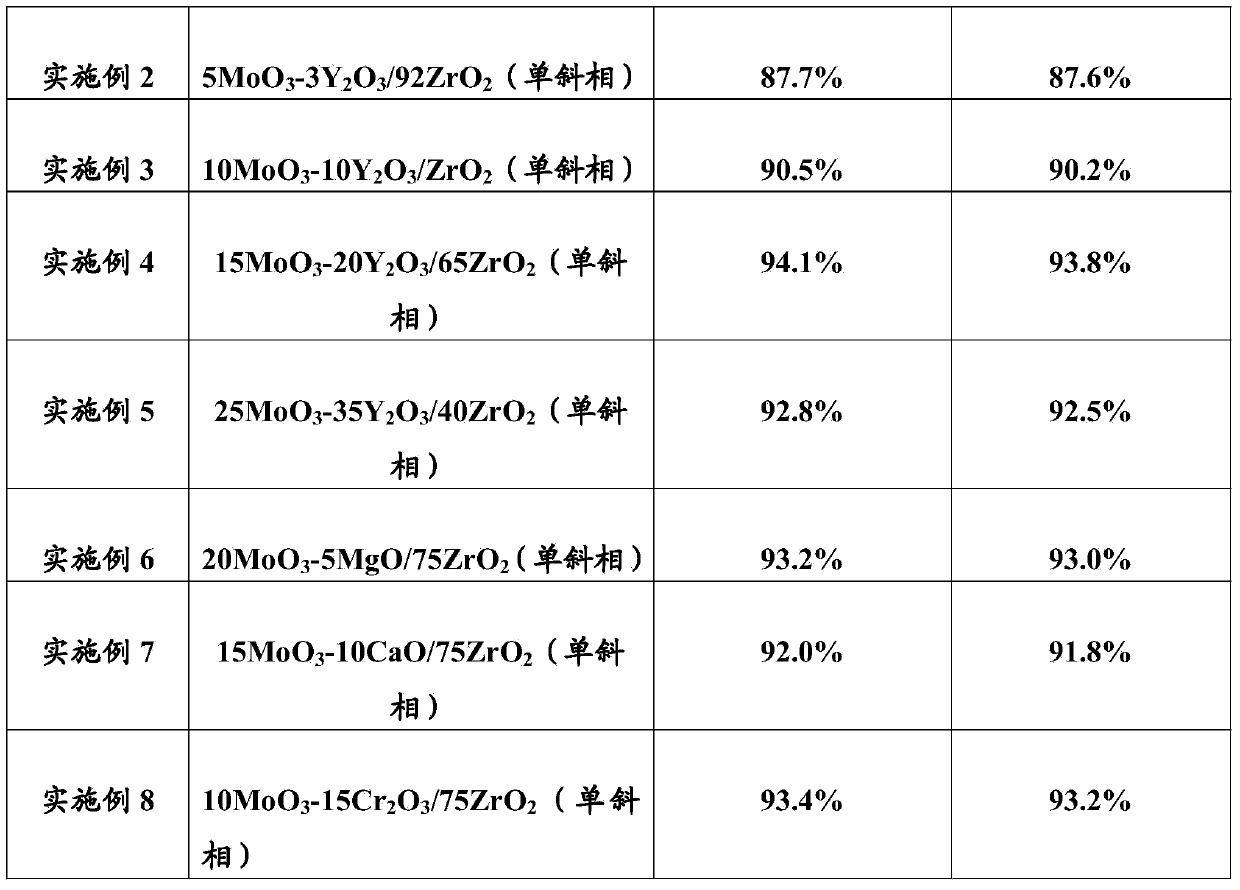
[0079] Example 2: Preparation of 5MoO by impregnation method 3 -3Y 2 o 3 / 92ZrO 2 (monoclinic) catalyst
[0080] (1) 2.54 grams of Y (NO 3 ) 3 .6H 2 O was dissolved in 70 g of deionized water and stirred to form an impregnation solution. Weigh 92 g of commercially available monoclinic ZrO 2 Carrier (with a specific surface area of 90m 2 / g), add it to the impregnation solution, stir vigorously for 2 hours to form a uniform suspension, evaporate the water to dryness with a rotary evaporator, and then put it in a 110°C drying oven to dry for 12 hours. Finally, Baked in a muffle furnace at 600 °C for 4 hours to obtain surface-loaded Y 2 o 3 Monoclinic ZrO 2 porous carrier.
[0081] (2) 6.1 grams of ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) be dissolved in 70 grams of deionized water, and be made into dipping solution through stirring. The surface load Y obtained in the above step (1) will be 2 o 3 Monoclinic ZrO 2 Add the porous carrier into the impre...
Embodiment 3
[0082] Example 3: Preparation of 10MoO by impregnation method 3 -10Y 2 o 3 / 80ZrO 2 (monoclinic) catalyst
[0083] (1) 8.48 grams of Y (NO 3 ) 3 .6H 2 O was dissolved in 60 g of deionized water and stirred to form an impregnation solution. Weigh 80 g of commercially available monoclinic ZrO 2 Carrier (with a specific surface area of 90m 2 / g), add it to the impregnation solution, stir vigorously for 2 hours to form a uniform suspension, evaporate the water to dryness with a rotary evaporator, and then put it in a 110°C drying oven to dry for 12 hours. Finally, Baked in a muffle furnace at 600 °C for 4 hours to obtain surface-loaded Y 2 o 3 Monoclinic ZrO 2 porous carrier.
[0084] (2) 12.26 grams of ammonium molybdate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) be dissolved in 60 grams of deionized water, and be made into dipping solution through stirring. The surface load Y obtained in the above step (1) will be 2 o 3 Monoclinic ZrO 2 Add the porous carrier into the i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com