A kind of preparation method of paper with antibacterial effect
A technology for paper and antibacterial drugs, applied in the field of paper preparation, can solve the problems of failure of antibacterial effect, loss, uneven distribution of antibacterial agents in antibacterial paper, etc., and achieve the effects of reducing production costs, reducing side effects, and overcoming the problem of loss of antibacterial drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
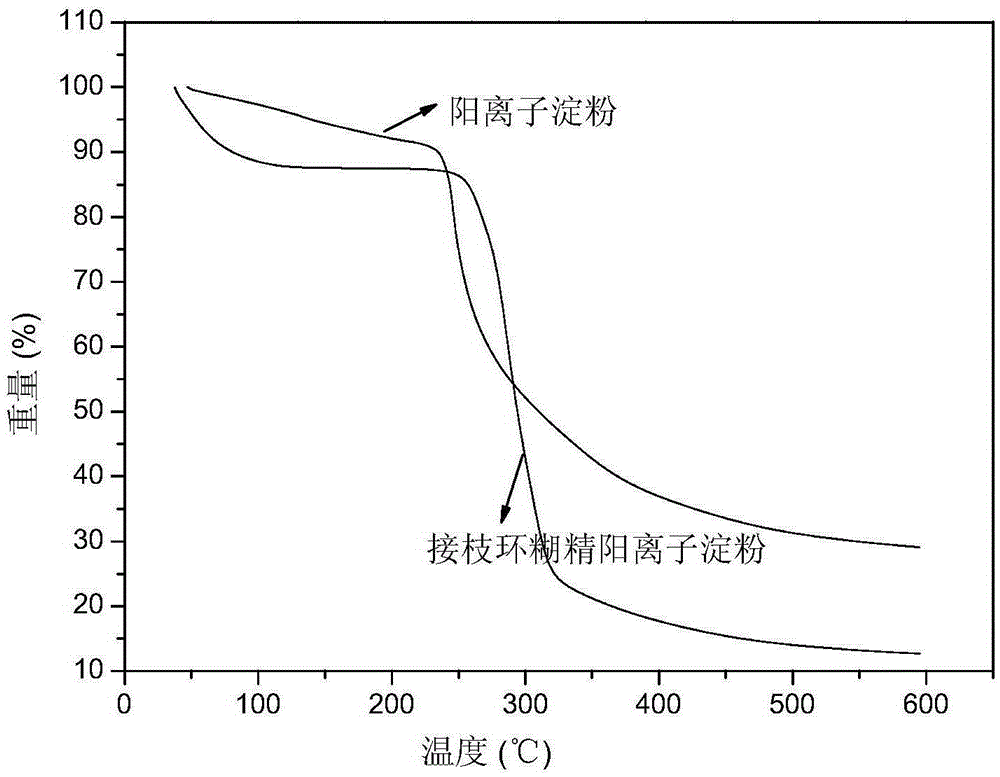
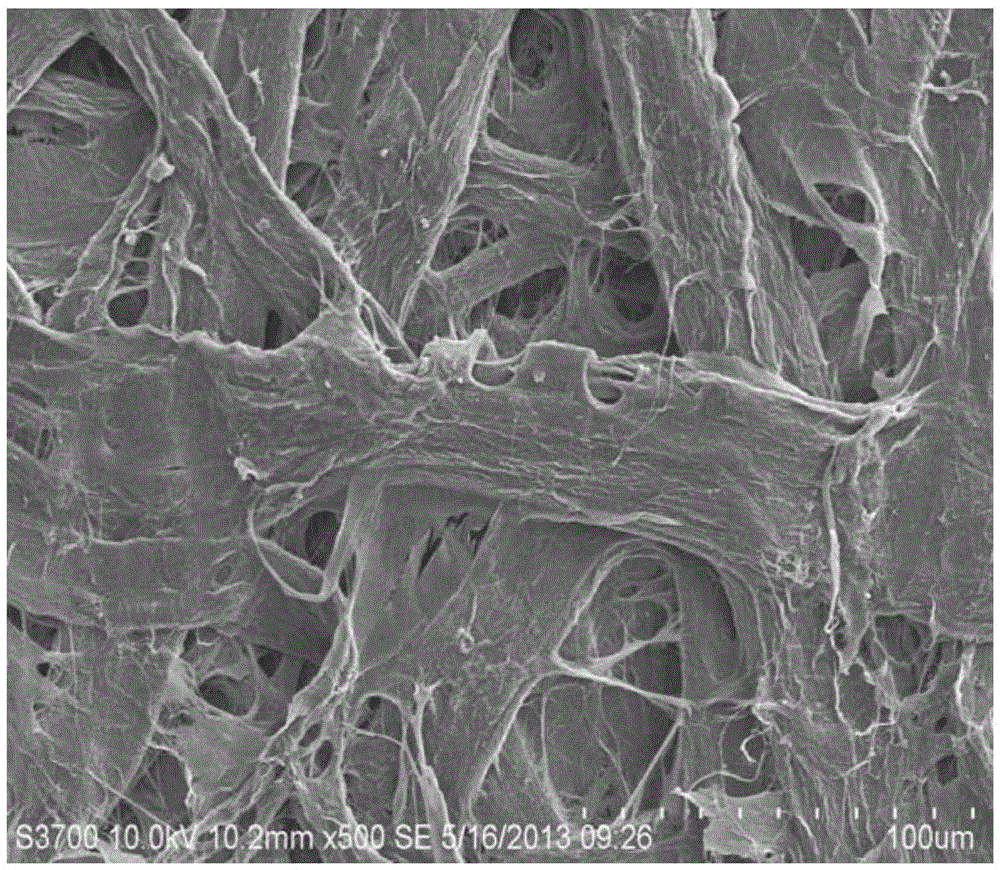
Image
Examples
Embodiment 1
[0028] (1) At room temperature, add 5g of β-cyclodextrin to 25ml of sodium hydroxide solution with a concentration of 300g / L, slowly add 1ml of epichlorohydrin solution, stir at 50°C for 20min, then add 2.5g of gelatinized replacement cationic starch with a density of 0.021, continue to stir for 2 hours, neutralize the solution with 1.5mol / L hydrochloric acid solution until it becomes neutral, add 50ml of ethanol solution, centrifuge at 5000r / min for 15min, freeze dry for 12h to obtain grafted cyclodextrin cationic starch
[0029] (2) Put 2 g of the prepared grafted cyclodextrin cationic starch in a mortar, add 4 g of deionized water to grind it evenly, add 200 mg of ciprofloxacin hydrochloride and then grind it for 20 min, then place it in a blast drying oven at 40 °C Drying in medium for 4h, followed by washing 3 times with deionized water, freeze-drying for 12h to obtain the grafted cyclodextrin cationic starch containing ciprofloxacin hydrochloride;
[0030] (3) Add 0%, 0....
Embodiment 2
[0034] (1) At room temperature, add 6g of β-cyclodextrin to 25ml of sodium hydroxide solution with a concentration of 400g / L, slowly add 2.42ml of epichlorohydrin solution, stir at 60°C for 30min, then add 2.5g of The final degree of substitution is 0.033 cationic starch, continue to stir for 3 hours, neutralize the solution with 1mol / L hydrochloric acid solution until it becomes neutral, add 50ml of ethanol solution, centrifuge at 4000r / min for 20min, freeze-dry for 12h to obtain grafted cyclodextrin cationic starch;
[0035] (2) Put 2 g of the prepared grafted cyclodextrin cationic starch in a mortar, add 6 g of water and grind it evenly, add 300 mg of levofloxacin hydrochloride and grind it thoroughly for 20 min, dry it in a blast drying oven at 50 °C for 3 h, and press Then wash 3 times with deionized water, and freeze-dry for 12h to obtain the grafted cyclodextrin cationic starch containing levofloxacin hydrochloride;
[0036] (3) Add 0%, 0.1%, 1%, and 2% of the grafted ...
Embodiment 3
[0041] (1) At room temperature, add 7.5g of β-cyclodextrin to 25ml of 400g / L sodium hydroxide solution, slowly add 2.42ml of epichlorohydrin solution, stir at 60°C for 20min, then add 2.5g of β-cyclodextrin The degree of substitution after melting is 0.043 cationic starch, continue to stir for 4 hours, neutralize the solution with 1.5mol / L hydrochloric acid solution until it becomes neutral, add 50ml of acetone solution, centrifuge at 5000r / min for 30min, freeze-dry for 12h to obtain the grafted ring Dextrin cationic starch;
[0042] (2) Put 2 g of the obtained grafted cyclodextrin cationic starch in a mortar, add 8 g of water and grind it evenly, add 400 mg of doxycycline hydrochloride, grind it thoroughly for 20 min, and dry it in a blast drying oven at 40 °C for 4 h , followed by washing with deionized water for 3 times, and freeze-drying for 12 hours to obtain the grafted cyclodextrin cationic starch containing doxycycline hydrochloride;
[0043](3) Add 0%, 0.1%, 1%, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com