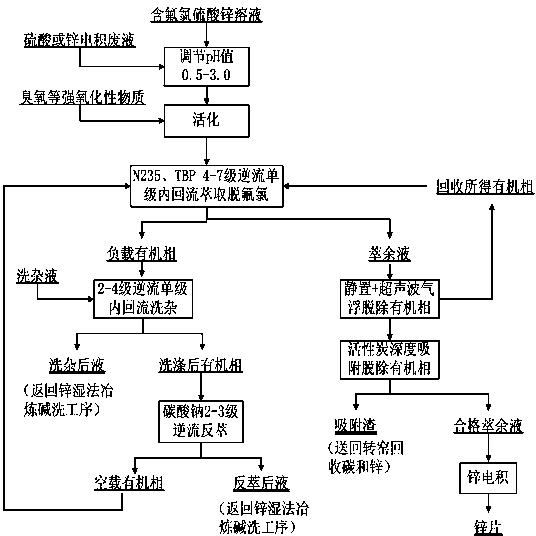
Activation extraction separation method for fluorine and chloride ions in zinc sulfate solution
A technology of zinc sulfate solution and ion activation, applied in the direction of improving process efficiency, etc., to achieve the effect of improving elution effect, reducing back extraction alkali consumption and crystallization scaling pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0033] (1) Adjusting pH and activating: Purify the qualified electrowinning solution (chemical composition: Zn 2+ 134.3g / L, F - 0.54g / L, Cl - 2.79 g / L, pH value 4.5-5.2, temperature 56°C) into the zinc electrowinning waste liquid in the zinc wet process system (chemical composition: Zn 2+ 49.5g / L, F - 0.19g / L, Cl - 0.47 g / L, H 2 SO 4 153.7g / L, temperature 35°C), the ratio of qualified electrowinning new solution and zinc electrowinning waste solution is (volume ratio) 20:1, and they are mixed evenly in the stirring tank. Zinc sulfate solution (chemical composition is: Zn 2+ 129.8g / L, F - 0.52g / L, Cl - 2.71 g / L, pH 1.0, temperature 26°C). Then pump the zinc sulfate solution after cooling into the self-made activation device, and pass into the activation agent ozone (O 3 ), ozone is produced from an ozone generator. The ozone flux is F in the zinc sulfate solution - 5 times the amount (mass ratio), the activation time is 1h, and the activation process is co...
example 2
[0039] (1) Adjusting pH and activating: Purify the qualified electrowinning solution (chemical composition: Zn 2+ 129.7g / L, F - 0.58g / L, Cl - 3.58 g / L, pH value 4.5-5.2, temperature 60°C) into the zinc electrowinning waste liquid in the zinc wet process system (chemical composition: Zn 2+ 47.5g / L, F - 0.18g / L, Cl - 0.50 g / L, H 2 SO 4 145.6g / L, temperature 34°C), the amount (volume) of zinc electrowinning waste solution is 3% of the volume of qualified electrowinning solution, and mixed evenly in the stirring tank. Zinc sulfate solution (chemical composition is: Zn 2+ 127.3g / L, F - 0.57g / L, Cl - 3.50 g / L, pH 2.0, temperature 25°C). Then pump the zinc sulfate solution after cooling into the self-made activation device, and pass into the activation agent ozone (O 3 ), ozone is produced from an ozone generator. The ozone flux is F in the zinc sulfate solution - The amount is 3.5 times (mass ratio), the activation time is 1.5h, and the activation process is co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com